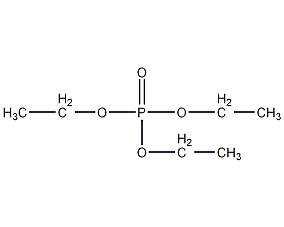
Structural formula
| Business number | 01N1 |
|---|---|
| Molecular formula | C6H15O4P |
| Molecular weight | 182 |
| label |
triethyl phosphate, triethylphosphate, Triethoxyphosphonium, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:78-40-0
MDL number:MFCD00009077
EINECS number:201-114-5
RTECS number:TC7900000
BRN number:1705772
PubChem number:24889379
Physical property data
1. Properties: Colorless, easy-flowing liquid with a slight fruity aroma.
2. Relative density (g/mL, 20/4℃): 1.06817
3. Relative vapor density (g/mL, air=1): 6.28
4. Melting point (ºC): -56.4
5. Boiling point (ºC, normal pressure): 210~220
6. Refractive index (25ºC): 1.4948
7. Flash point (ºC, opening): 117
8. Autoignition point or ignition temperature (ºC ): 451.7
9. Vapor pressure (mmHg, 40ºC): 1
10. Saturated vapor pressure (kPa, 39ºC): 0.13
11. Evaporation Heat (KJ/mol): 57.36
12. Heat of formation (KJ/mol): 1248.5
13. Heat of combustion (KJ/mol): 4117.3
14. Solubility (%, 25ºC, water): 100
15. Explosion upper limit (%, V/V): 10.0
16. Lower explosion limit (%, V/V ): 1.7
17. Solubility: Soluble in organic solvents such as alcohol and ether, and miscible with water in any proportion.
Toxicological data
1. Acute toxicity: rat oral LD50: >800mg/kg; mouse oral LD50: 1500mg/kg
2. Mild irritation to the skin. At relatively high doses a narcotic phenomenon and significant muscle relaxation are produced. Inhibition of brain cholinesterase in in vitro assays.
Ecological data
Slightly hazardous to water, avoid contact of undiluted or large quantities of product with groundwater, waterways or sewage systems.
Molecular structure data
1. Molar refractive index: 41.95
2. Molar volume (cm3/mol): 170.8
3. Isotonic specific volume (90.2K ): 401.9
4. Surface tension (dyne/cm): 30.6
5. Polarizability (10-24cm3): 16.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Tautomers.�
This product is a high boiling point solvent and a plasticizer for rubber and plastics. Also a catalyst. It is also used as a raw material for preparing pesticides and insecticides. and as an ethylation reagent for ketene production. In Japan, 70% of this product is used as catalyst and about 20% is used as solvent. The main uses are as follows: (1) Catalyst xylene isomer catalyst; polymerization catalyst for olefins; catalyst for manufacturing tetraethyl lead; catalyst for manufacturing carbodiimide; catalyst for replacement reaction of trialkyl boron and olefins; using acetic acid at high temperature Catalyst for dehydration to produce ketene; catalyst for polymerization of styrene and conjugated diene compounds; if used in the polymerization of terephthalic acid and ethylene glycol, it can prevent fiber discoloration. (2) Solvents: solvents for cellulose nitrate and cellulose acetate; solvents used to maintain the life of organic peroxide catalysts; solvents for dispersing fluorinated ethylene; peroxide agents used as curing catalysts for polyester resins and epoxy resins and thinner. (3) Stabilizer: Chlorine pesticides and stabilizers; stabilizers for phenolic resins; solid agents for sugar alcohol resins. (4) For synthetic resins, curing agent for xylenol-formaldehyde resin; softener for phenolic resin used in shell molds; softener for vinyl chloride; plasticizer for vinyl acetate polymer; flame retardant for polyester resin .

 微信扫一扫打赏
微信扫一扫打赏

