Background and overview[1]
Indanone and its derivatives are a very important class of organic compounds. Many natural products and drug molecules contain indenone structural units. In addition, indenone derivatives are also important organic synthesis intermediates and are widely used in the synthesis of many drugs. Among the numerous indenone derivatives, many compounds with an aryl group at the benzyl position of the indene ring show various interesting biological activities. In view of the important applications of indanone compounds in organic chemistry and medicinal chemistry, their synthesis methods have also received widespread attention.
Indenone compounds are often produced from phenylpropionic acid and its derivatives as raw materials and are catalyzed by various strong acids for ring closure, such as chlorosulfonic acid, trifluoromethanesulfonic acid, trifluoroacetic acid, polyphosphoric acid, etc. The environment is polluted, the reaction conditions are harsh, and there is a lack of stable and reliable synthesis methods with a wide range of substrates and mild conditions. In recent years, great progress has been made in the synthesis of benzyl-substituted derivatives of the indene ring catalyzed by transition metals from α,β-unsaturated carbonyl compounds. 3-Phenyl-1-indanone can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of phenyl-1-indanone is as follows:
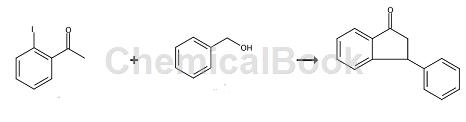
The specific steps are as follows: Under the protection of high-purity nitrogen, add 0.05mmol of isonuclear ruthenium-palladium bicyclic metal compound 1, 1mmol of o-iodoacetophenone, 2.5mmol of benzyl alcohol, 2mmol of potassium hydroxide and 3 ml of dioxane, replace the reaction tube with nitrogen three times, then heat to 110°C in an oil bath under magnetic stirring, and reflux the reaction for 30 hours. Remove the oil bath and cool the water bath to room temperature; add 3 ml of water to the reaction solution, extract three times with 5 ml of dichloromethane, combine the organic phases and dry with anhydrous MgSO4 for 30 minutes, filter, and concentrate the filtrate with a rotary evaporator. Methane/petroleum ether mixture was used as a developing agent and separated by silica gel thin layer chromatography to obtain the pure product 3-phenyl-1-indanone.
The NMR analysis data of this compound are as follows: 1HNMR: δ=7.81(d, 1H, Ph-H), 7.56(t, 1H, Ph-H), 7.43(t, 1H, Ph-H), 7.24- 7.35 (m, 4H, Ph-H), 7.14 (m, 2H, Ph-H), 4.57 (dd, 1H, CH), 3.24 (dd, 1H, CH2), 2.73 (dd, 1H, CH2).
Main reference materials
[1] CN201310076776.7 Heteronuclear ruthenium and palladium bicyclic metal compounds and their preparation methods and applications

 微信扫一扫打赏
微信扫一扫打赏

