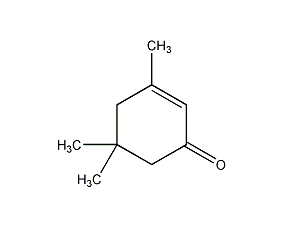
Structural formula
| Business number | 01N7 |
|---|---|
| Molecular formula | C9H14O |
| Molecular weight | 138.21 |
| label |
3,5,5-Trimethyl-2-cyclohexen-1-one, 3,5,5-Trimethyl-2-cyclohexene-1-one, high boiling point solvents, Thinner |
Numbering system
CAS number:78-59-1
MDL number:MFCD00001584
EINECS number:201-126-0
RTECS number:GW7700000
BRN number:1280721
PubChem number:24881360
Physical property data
1. Properties: Colorless liquid, low volatility, with a camphor-like odor.
2. Relative density (g/mL, 20/20℃): 0.9255
3. Relative density (20℃, 4℃): 0.9229
4. Melting point (ºC): -8.1
5. Boiling point (ºC, normal pressure): 215.3
6. Relative density (25℃, 4℃): 0.9185
7. Refractive index (n18D): 1.4766
8. Flash point (ºC, Opening): 84
9. Viscosity (mPa·s, 20ºC): 2.62
10. Fire point (ºC): 462
11. Refractive index at room temperature (n25): 1.4750
12. Heat of evaporation (KJ/mol): 48.15
13. Heat of combustion (KJ/mol): 5272
14. Liquid phase standard hot melt (J·mol-1·K-1): 275.7
15. Critical Pressure (KPa): Uncertain
16. Specific heat capacity (KJ/(kg·K), 15ºC, constant pressure): 1.78
17. Explosion upper limit (%, V/V ): 3.8
18. Lower explosion limit (%, V/V): 0.84
19. Solubility: miscible with most organic solvents and most nitrocellulose paints. It has high dissolving ability for cellulose esters, cellulose ethers, greases, natural and synthetic rubbers, and resins, especially for nitrocellulose, vinyl resin, alkyd resin, melamine resin, polystyrene, etc. Dissolves 1.2% in water at 20°C; water dissolves 4.3% in isophorone.
Toxicological data
It is of low toxicity. The vapor is more toxic than simple aliphatic ketones, but the vapor pressure is low at room temperature, so it is less harmful. Rat test results show that isophorone is one of the most toxic ketones and can cause kidney disorders and damage the cornea of the eye. People may feel irritable after contact. When the vapor concentration reaches 141mg/m3 or above, it will cause irritation to the eyes and nose. It has strong degreasing effect and should avoid contact with skin. Non-corrosive to metals. The maximum allowable concentration in the workplace is 141 mg/m3.
Ecological data
None yet
Molecular structure data
1�� Molar refractive index: 41.43
2, Molar volume (cm3/mol): 152.6
3, Isotonic specific volume (90.2K): 346.2
4. Surface tension (dyne/cm): 26.4
5. Polarizability (10-24cm3) :16.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 187
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is a flammable liquid, but it evaporates slowly and is difficult to catch fire.
2. Chemical properties: dimer is generated under light; 3,5-xylenol is generated when heated to 670~700℃; 4,6,6-trimethyl-1 is generated when oxidized by air , 2-cyclohexanedione; when treated with fuming sulfuric acid, isomerization and dehydration occur; no addition reaction occurs with sodium bisulfite, but can add with hydrocyanic acid; 3,5 is formed when hydrogenated ,5-trimethylcyclohexanol.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and mainstream smoke.
Storage method
This product should be kept sealed.
Synthesis method
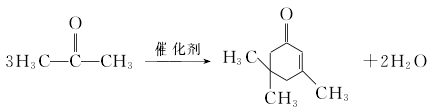
1. Obtained from the polymerization of 3 molecules of acetone.
2.The mesityl oxide method uses mesityl oxide and ethyl acetoacetate under the action of a catalyst to prepare isophorone through cyclization and hydrolysis. urone. The advantage of this process route is that it is carried out under normal pressure and lower temperature. Therefore, it has mild conditions, simple operation and low equipment investment. The disadvantage is that due to the high price of raw materials and high product cost, it is difficult to promote and apply, so this method has now been eliminated.
The acetone condensation method uses an alkaline catalyst and high temperature conditions to condense three molecules of acetone to generate one molecule of isophorone:
span>
The method of synthesizing isophorone from acetone can be divided into two types according to the contact state. One is the heterogeneous catalytic condensation method with a solid catalyst, and the other is a pressurized liquid phase in an alkaline solution. Condensation method.
① Solid catalyst heterogeneous catalytic condensation method in which the catalyst is made of magnesium aluminate, zinc oxide, bismuth oxide, calcium oxide, calcium hydroxide, and diatomite Under the action of anionic catalyst, etc.), acetone is condensed at about 300°C to produce isophorone.
②Liquid phase condensation method This method is divided into two methods: intermittent and continuous. The continuous method is conducive to reducing costs and increasing output. It is easy to realize automatic control and improve working conditions. At present, the continuous method is mostly used abroad. The process flow of the continuous liquid phase condensation method of isophorone is as follows: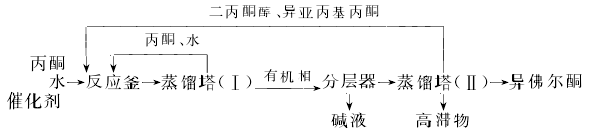
Add a certain amount of acetone, water, and catalyst into the reactor, and control the reaction temperature at 150 to 300 ℃, pressure 3.0~3.5MPa, time 1.5~2h. After the reaction is completed, add the material to the 1# distillation tower for normal pressure distillation, steam out unreacted acetone and water, and return to the reaction kettle after condensation to continue participating in the reaction. Then put the material into the layerer for phase separation, and separate the lower layer of alkali liquid for treatment. The upper layer is added to the 2# distillation tower. The steamed diacetone alcohol and mesityl acetone can be sold as products, or can be returned to the reaction kettle to continue participation. reaction. The total yield of isophorone obtained under this condition is 60% to 70%, the yield of diacetone alcohol is 16%, the yield of mesityl oxide is 7%, and the high boiling matter content is about 3% to 7%. The acetone conversion rate is 30% to 40%.
Refining method Isophorone is obtained by condensation of acetone and contains unreacted acetone, diacetone alcohol, mesityl oxide and other impurities. It is generally refined by vacuum distillation. Or add 0.05%~0.1% p-toluenesulfonic acid to crude isophorone for vacuum distillation. It can also be washed with 5% sodium carbonate aqueous solution and then refined by distillation under reduced pressure.
3. Tobacco: BU, 56; BU, 14; FC, 9, 18, 40; BU, OR, 18.
Purpose
1. This product is a high boiling point solvent. Mainly used in pesticides, paints and can coatings. It is a high boiling point solvent for nitrocellulose spray paint and synthetic resin coatings. Special coatings are used as thinners. Mixed with methyl isobutyl ketone to dissolve phenolic and epoxy resins. It is also used as a raw material for the manufacture of 3,5,5-trimethylcyclohexanol and 3,5-dimethylphenol.
2.It is a good high-boiling point ketone solvent with strong solubility. It is widely used in industries such as adhesives, plastics, pesticides, medicines, coatings, and energetic polymer solid propellants. It can be used in phenol resin adhesives, shoe adhesives, tape adhesives, casting sand adhesives, resins, inks and other industries. It is a diluent for epoxy resin, phenolic resin, fluororesin, nitro spray paint, acrylic resin baking curing paint, tank inner wall paint, ink and plastic paint. Because it has the structure of a conjugated unsaturated ketone, the double bonds can undergo further reactions such as addition, hydrogenation, and phosgenation to obtain important products such as alcohols, acids, amines, esters, and isocyanates. In addition, the excellent solubility of isophorone can be used as a solvent for emulsifying pesticides, which can increase the solubility of pesticides by more than 20%.
Thinner for nitrocellulose spray paint, acrylic resin bake-cured paint, tank inner wall paint, ink and plastic paint. Because it has the structure of a conjugated unsaturated ketone, the double bonds can undergo further reactions such as addition, hydrogenation, and phosgenation to obtain important products such as alcohols, acids, amines, esters, and isocyanates. In addition, the excellent solubility of isophorone can be used as a solvent for emulsifying pesticides, which can increase the solubility of pesticides by more than 20%.

 微信扫一扫打赏
微信扫一扫打赏

