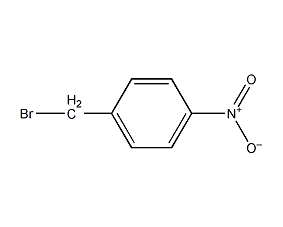
Structural formula
| Business number | 02H6 |
|---|---|
| Molecular formula | C7H6BrNO2 |
| Molecular weight | 216 |
| label |
p-nitrobenzyl bromide, 5-nitrobenzyl bromide, p-Nitrophenyl bromide, 4-nitrobenzyl bromide, 1-(Bromomethyl)-4-nitro-benzen, 4-(Bromomethyl)nitrobenzene |
Numbering system
CAS number:100-11-8
MDL number:MFCD00007373
EINECS number:202-820-6
RTECS number:XS7967000
BRN number:742796
PubChem number:24897481
Physical property data
1. Properties: white or light yellow needle-like crystals, tear-inducing. [1]
2. Melting point (℃): 98~100[2]
3. Flash point (℃) ): >100[3]
4. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol and ether. [4]
Toxicological data
1. Acute toxicity[5] LD50: 56 mg/kg (mouse vein)
2. Irritation No information yet
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 45.44
2. Molar volume (cm3/mol): 130.7
3. Isotonic specific volume (90.2K ): 351.7
4. Surface tension (dyne/cm): 52.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.01
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 138
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[6] Stable
2. Incompatible substances[7] Alkali, amines, strong oxidants, alcohols, strong reducing agents
3. Conditions to avoid contact [8] Heat, Moist air
4. Polymerization hazard[9] Does not polymerize
5. Decomposition products[ 10] Nitrogen oxides, hydrogen bromide
Storage method
Storage Precautions[11] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, reducing agents, alkalis, amines, alcohols, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Preparation method:
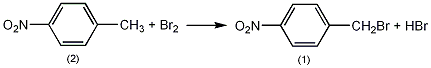
Add 150g (1.1mol) of p-nitrotoluene (2) into a reaction bottle equipped with a stirrer, thermometer, reflux condenser (connected to an air conduit to absorb hydrogen bromide) and a dropping funnel, and heat in an oil bath while stirring to 145~150℃, slowly add 184g (1.15mol) of bromine dropwise, and finish the addition in 2 hours. After the addition is complete, continue the reaction for 10 minutes. After cooling slightly, pour into 2L of hot petroleum ether (80~100℃), add activated carbon, and reflux for 10 minutes. Filter while hot, cool to about 20°C, filter out the crystals, wash with petroleum ether and dry to obtain 150g of crude p-nitrobenzyl bromide (1), mp95~97°C. Recrystallized in petroleum ether, mp98~99℃, output 135g, yield 57%. [13]
Purpose
Used as organic analysis reagents and dye intermediates. [12]

 微信扫一扫打赏
微信扫一扫打赏

