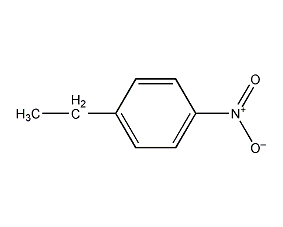
Structural formula
| Business number | 02H7 |
|---|---|
| Molecular formula | C8H9NO2 |
| Molecular weight | 151.17 |
| label |
p-Ethylnitrobenzene, p-Nitroethylbenzene, 1-Ethyl-4-nitrobenzene, p-nitroethylbenzene |
Numbering system
CAS number:100-12-9
MDL number:MFCD00007385
EINECS number:202-821-1
RTECS number:DH5600000
BRN number:1862416
PubChem ID:None
Physical property data
1. Properties: colorless oily liquid [1]
2. Melting point (℃): -12.3[2]
3. Boiling point (℃): 245~246[3]
4. Relative density (water=1): 1.12[4]
5. Saturated vapor pressure (kPa): 3.06 (135°C) [5]
6. Octanol/water partition coefficient: 3.03 [6]
7. Flash point (℃): >112[7]
8. Solubility: insoluble Soluble in water, soluble in most organic solvents such as ethanol and ether. [8]
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[9] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 42.34
2. Molar volume (cm3/mol): 134.0
3. Isotonic specific volume (90.2K ): 339.3
4. Surface tension (dyne/cm): 41.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 134
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[10] Stable
2. Incompatible substances[11] Strong oxidants, strong reducing agents, strong bases, strong acids
3. Conditions to avoid contact[12] Heat
4. Hazards of aggregation[13] No aggregation
5.�Decomposition products[14] Nitrogen oxides
Storage method
Storage Precautions[15] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Originated from the nitration of ethylbenzene.
Purpose
Used in organic synthesis. [16]

 微信扫一扫打赏
微信扫一扫打赏

