Background and overview[1]
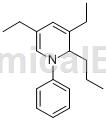
3,5-diethyl-1,2-dihydro-1-phenyl-2-propylpyridine can be used as a pharmaceutical synthesis intermediate. If 3,5-diethyl-1,2-dihydro-1-phenyl-2-propylpyridine is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and wash with soap Rinse the skin thoroughly with water and clean water. If you feel discomfort, seek medical treatment; if the eye contact occurs, separate the eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 3,5-diethyl-1,2-dihydro-1-phenyl-2-propylpyridine (DHP) is as follows:
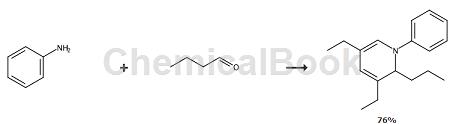
Method 1: A 1000ml four-neck flask is equipped with a mechanical stirrer, a reflux condenser, a thermometer and an addition funnel. Cool the flask with an ice-water bath if necessary. To the flask were added 86 grams of deionized water, 9.8 grams (0.16 moles) of acetic acid, and 216 grams (3.0 moles) of butyraldehyde. While cooling and stirring, 60 g (0.64 mol) of aniline were added over a period of 35 minutes while maintaining the reaction temperature at 20°C. The reaction mixture was stirred below 25°C for one hour. The reaction mixture was then heated to 75°C and kept warm for two hours. Finally, the reaction mixture was heated to reflux (~90°C) and held for five hours. The reaction mixture was cooled and the layers separated. The upper organic layer was distilled under reduced pressure through a 14″ packed column. The fraction taken out at a top temperature of 140-143°C and a pressure of 5 mmHg weighed 104 g. Analysis showed that it contained 73% DHP, with a total yield of 46 %.
Method 2: Under stirring and cooling, add 433 g (6.0 mol) butyraldehyde, 120 g (1.3 mol) aniline and 9.8 g acetic acid (0.16 mol) into the flask. The mixture was maintained at 20-25°C during the addition of each reactant, after which the reaction temperature slowly increased to 40°C. The reaction mixture was stirred at 40°C for one hour and then heated to 75°C for five hours. A 10% aqueous sodium carbonate solution (100 ml) was added to the flask and the reaction mixture was heated to reflux for a further five hours. The reaction mixture was cooled and the layers separated. The upper organic layer was distilled under reduced pressure through a 14″ packed column. The product fraction obtained at a top temperature of 140-143°C and a pressure of 4 mmHg weighed 219 g. Analysis showed that it contained 89% DHP, which is equivalent to The overall chemical yield is 59%.
Main reference materials
[1] CN101213176

 微信扫一扫打赏
微信扫一扫打赏

