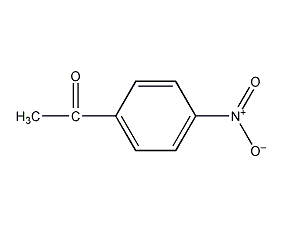
Structural formula
| Business number | 02HE |
|---|---|
| Molecular formula | C8H7NO3 |
| Molecular weight | 165.15 |
| label |
1-(4-nitrophenyl)ethanone, 4-nitroacetophenone, p-nitroacetophenone, p-nitroacetophenone, 1-(4-Nitrophenyl)-ethanon, 1-Acetyl-4-nitrobenzene, 4’-Nitro-acetophenon, 4-Acetylnitrobenzene, 4-Nitrophenylmethylketone, Acetophenone,4’-nitro-, Ethanone, 1-(4-nitrophenyl)-, Ethanone,1-(4-nitrophenyl)-, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:100-19-6
MDL number:MFCD00007355
EINECS number:202-827-4
RTECS number:AM9627000
BRN number:607777
PubChem ID:None
Physical property data
1. Properties: The pure product is light yellow crystals or needle-shaped crystals.
2. Density (g/mL, 20℃): 0.86
3. Relative vapor density (g/mL, air=1): 5.6
4. Melting point (ºC): 75-78
5. Boiling point (ºC, normal pressure): 202
6. Boiling point (ºC, 0.6mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 149
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20ºC):
12. Saturated vapor pressure (kPa, ºC) : Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined Determined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot ethanol, ether and benzene, insoluble in water.
Toxicological data
Mutagenicity: Mutant microorganism test: bacteria-Salmonella typhimurium, 220μg/plate; Mutant microorganism test: bacteria-Salmonella typhimurium, 100μg/plate; DNA repair test: Bacillus subtilis, 500μg/disc;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 42.82
2. Molar volume (cm3/mol): 132.8
3. Isotonic specific volume (90.2K ): 347.9
4. Surface tension (dyne/cm): 47.1
5. Dielectric constant: 2.30
6. Dipole moment (10-24cm3):
7. Polarizability: 16.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 189
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
The toxicity is unknown. Production equipment should be sealed and operators should wear protective equipment.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
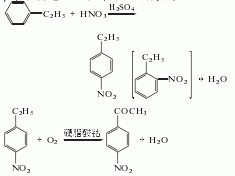
1. Nitroethylbenzene is obtained by nitrating ethylbenzene with mixed acid at 30-35°C. After distillation, p-nitroethylbenzene and co-product o-nitroethylbenzene are obtained. In the presence of catalyst cobalt stearate, p-nitroethylbenzene is oxidized with air at 140-150°C and 0.2MPa pressure to obtain p-nitroacetophenone. The reaction product is washed with water, neutralized, dehydrated by centrifugation, and dried to obtain the finished product. Raw material consumption quota: ethylbenzene 2519kg/t, nitric acid 1535kg/t, sulfuric acid 697kg/t, hydrochloric acid 12kg/t, soda ash 81kg/t, caustic soda 111kg/t, ethanol 100kg/t, methanol 70kg/t.
2. p-Nitrobenzene Acid chloride method.
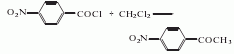
Purpose
Intermediates used in organic synthesis and pharmaceutical synthesis (such as synmycin, chloramphenicol). Also used in the synthesis of pesticides, dyes, and spices.

 微信扫一扫打赏
微信扫一扫打赏

