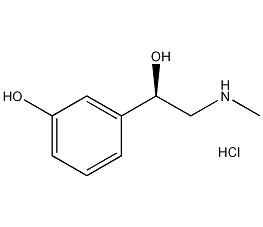
Structural formula
| Business number | 01C1 |
|---|---|
| Molecular formula | C9H14ClNO2 |
| Molecular weight | 203.67 |
| label |
phenylephrine hydrochloride, (R)-Phenylephrine hydrochloride, HOC6H4CH(CH2NHCH3)OH·HCl, (R)-(−)-3-Hydroxy-α-(methylaminomethyl)benzyl alcohol hydrochloride, (R)-(−)-1-(3-Hydroxyphenyl)-2-methylaminoethanol hydrochloride |
Numbering system
CAS number:61-76-7
MDL number:MFCD00012605
EINECS number:200-517-3
RTECS number:DO7525000
BRN number:4158948
PubChem number:24277799
Physical property data
1. Character:White or off-white crystalline powder
2. Density (g/mL,25/4℃): No��determined
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):143-145(lit.) (The melting point of the left-handed free base is169-172℃)
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:-45.5 ° (C=1, H2O)
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º):-47 º (c=2, H2O)
10. Autoignition point or ignition temperature (ºC): Unsure
Topological molecular polar surface area (TPSA): 52.5
6. Heavy atoms Quantity: 13
7. Surface charge :0
8. Complexity :130
9. Isotope atomic number:0
10. Determine the number of atomic stereocenters:1
11. Uncertain number of atomic stereocenters:0
12. Determine the number of stereocenters of chemical bonds:0
13. Uncertain number of chemical bond stereocenters:0
14. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry place.
Synthesis method
Meta-hydroxyethyl ketone is benzoylated, brominated, Amination, hydrolysis, catalytic hydrogenation to obtain meta-hydroxyl-α-Methylaminomethylbenzyl alcohol (racemate), reusedd-Tartaric acid is separated to obtain left-handed free base. Suspend the free base in absolute ethanol and add hydrogen chloride–Ethanol solution topH=4, heat to dissolve, add activated carbon to decolorize and filter, add anhydrous ether to the filtrate, and freeze to precipitate crystals. Filter, wash the crystal with a small amount of acetone, and dry to obtain phenylephrine hydrochloride.
Purpose
Adrenergic drugs. It has a constrictive effect on local blood vessels in the body. It is used to extend the time of local anesthesia during surgical procedures and to reduce swelling and inflammation of nasal mucosa congestion.
op-alt: auto; mso-margin-bottom-alt: auto; mso-list: l0 level1 lfo1; tab-stops: list 36.0pt” align=left>12. Determine the number of stereocenters of chemical bonds:0
13. Uncertain number of chemical bond stereocenters:0
14. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry place.
Synthesis method
Meta-hydroxyethyl ketone is benzoylated, brominated, Amination, hydrolysis, catalytic hydrogenation to obtain meta-hydroxyl-α-Methylaminomethylbenzyl alcohol (racemate), reusedd-Tartaric acid is separated to obtain left-handed free base. Suspend the free base in absolute ethanol and add hydrogen chloride–Ethanol solution topH=4, heat to dissolve, add activated carbon to decolorize and filter, add anhydrous ether to the filtrate, and freeze to precipitate crystals. Filter, wash the crystal with a small amount of acetone, and dry to obtain phenylephrine hydrochloride.
Purpose
Adrenergic drugs. It has a constrictive effect on local blood vessels in the body. It is used to extend the time of local anesthesia during surgical procedures and to reduce swelling and inflammation of nasal mucosa congestion.
The crystals are washed with a small amount of acetone and dried to obtain phenylephrine hydrochloride.
Purpose
Adrenergic drugs. It has a constrictive effect on local blood vessels in the body. It is used to extend the time of local anesthesia during surgical procedures and to reduce swelling and inflammation of nasal mucosa congestion.

 微信扫一扫打赏
微信扫一扫打赏

