Background and overview[1]
3-(1-pyrrolidinyl)benzaldehyde can be used as a pharmaceutical synthesis intermediate.
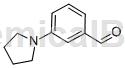
Preparation[1]
The preparation of (1-pyrrolidinyl)benzaldehyde is divided into the following steps:
Step 1: Synthesis of 2-(3-bromophenyl)-1,3-dioxolane (2):
To a stirred solution of 3-bromobenzaldehyde (500 mg, 2.70 mmol) in toluene (10 mL) under an argon atmosphere was added pTSA (catalytic amount) and ethane-1,2-diol (0.5 mL) at room temperature. , 8.10mmol); heat to 120°C and stir for 3 hours. The reaction was monitored by TLC; after the reaction was complete, the reaction mixture was quenched with ice-cold water (20 mL) and extracted with EtOAc (2 × 30 mL). The combined organic extracts were washed with saturated NaHCO solution (20 mL), dried over sodium sulfate, filtered and concentrated in vacuo. Crude 2-(3-bromophenyl)-1,3-dioxolane (550 mg, 89%), colorless liquid. The crude product was carried forward to the next step without further purification.
1H-NMR (CDCl3, 400MHz): δ7.64 (s, 1H), 7.50 (d, 1H), 7.41 (d, 1H), 7.18-7.17 (m, 1H), 5.80 (s, 1H) , 4.14(t,2H), 4.06(t,2H).
Step 2 Synthesis of 1-(3-(1,3-dioxolane-2-yl)phenyl)pyrrolidine:
To a stirred solution of 2-(3-bromophenyl)-1,3-dioxolane (0.3 g, 1.32 mmol) in toluene (15) at room temperature was added pyrrolidine (93 mg , 1.32mmol), Pd(dba)3 (24mg, 0.026mmol), BINAP (21mg, 0.033mmol) and NaOtBu (190mg, 1.98mmol) and degassed with argon. The resulting reaction mixture was heated to 70°C over 15 minutes and stirred for 16 hours; the progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature, filtered through a pad of Celite, and the filtrate was concentrated under reduced pressure to obtain crude product. The crude material was purified by silica gel column chromatography. 1-(3-(1,3-dioxolane-2-yl)phenyl)pyrrolidine (0.15 g, 52%) as a light yellow liquid.
1H-NMR (CDCl3, 400MHz): δ7.24-7.21 (m, 1H), 6.78 (d, 1H), 6.65 (s, 1H), 6.54 (d, 1H), 5.79 (s, 1H) , 4.14-3.98 (m, 4H), 3.32 (t, 4H), 1.98 (t, 4H); TLC: 5% EtOAc/Hexane (R f: 0.2).
Step 3: Synthesis of 3-(pyrrolidin-1-yl)benzaldehyde:
To a stirred solution of 1-(3-(1,3-dioxolane-2-yl)phenyl)pyrrolidine (0.15g, 0.68mmol) in THF (5mL) under nitrogen atmosphere, Add 1N HCl (3 mL) at 0 °C. The resulting reaction mixture was warmed to room temperature and stirred for 4 hours. The progress of the reaction was monitored by TLC; the volatiles were evaporated under reduced pressure and the resulting residue was diluted with EtOAc and washed with bicarbonate solution. The separated organic layer was dried over sodium sulfate, filtered and concentrated in vacuo to give crude product. The crude material was purified by silica gel column chromatography. 3-(pyrrolidin-1-yl)benzaldehyde (0.11 g, 92%), colorless liquid.
1H-NMR (CDCl3, 500MHz): δ9.94 (bs, 1H), 7.37 (t, 1H), 7.16-7.14 (m, 1H), 7.04 (d, 1H), 6.84-6.81 (m, 1H), 3.37 (t, 4H), 2.08 (t, 4H); TLC: 10% EtOAc/Hexane (R f: 0.4).
Main reference materials
[1] PCT Int. Appl., 2009038411, 26 Mar 2009

 微信扫一扫打赏
微信扫一扫打赏

