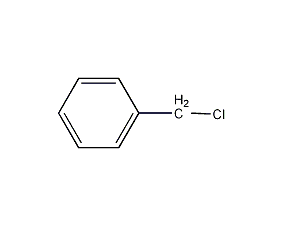
Structural formula
| Business number | 02HX |
|---|---|
| Molecular formula | C7H7Cl |
| Molecular weight | 126.59 |
| label |
Benzyl chloride; α-chlorotoluene;, 1-Chloromethylbenzene,α-Chlorotoluene, Aromatic halogen derivatives |
Numbering system
CAS number:100-44-7
MDL number:MFCD00000889
EINECS number:202-853-6
RTECS number:XS8925000
BRN number:471308
PubChem ID:None
Physical property data
1. Properties: colorless to yellow liquid with unpleasant pungent odor. [1]
2. Melting point (℃): -48~-39[2]
3. Boiling point ( ℃): 175~179[3]
4. Relative density (water=1): 1.10[4]
5. Relative vapor density (air=1): 4.36[5]
6. Saturated vapor pressure (kPa): 2.93 (78℃)[6]
7. Heat of combustion (kJ/mol): -3705.2[7]
8. Critical pressure (MPa): 3.91[8]
9. Octanol/water partition coefficient: 2.3[9]
10. Flash point (℃) : 67 (CC); 74 (OC) [10]
11. Ignition temperature (℃): 585[11]
12. Explosion upper limit (%): 14[12]
13. Explosion lower limit (%): 1.1[13]
14. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, chloroform, and ether. [14]
15. Viscosity (mPa·s, 20ºC): 1.38
16. Viscosity (mPa·s, 30ºC): 1.175
17. Heat of evaporation (KJ/mol, 25ºC): 51.1
18. Relative density (20℃, 4℃): 1.0127
19. Refractive index at room temperature (n25): 1.538920
20. Relative density (25℃, 4℃): 1.089830
21. Solubility parameter (J·cm-3)0.5: 20.123
22. van der Waals area (cm 2·mol-1): 8.450×109
23. van der Waals volume (cm 3·mol-1): 67.690
24. Gas phase standard claims heat (enthalpy) (kJ·mol-1): 18.9
25. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -32.6
26. Liquid phase standard hot melt ( J·mol-1·K-1):187.4
Toxicological data
1. Acute toxicity[15]
LD50: 1231mg/kg (rat oral)
LC50: 778mg/m3 (rat inhalation, 2h)
2. Irritation No information available
3. Mutagenicity[16] Microbial mutagenicity: Salmonella typhimurium 600μmol/dish; Escherichia coli 10mg/L . Microbial mutagenicity: Salmonella typhimurium 7900nmol/dish. DNA damage: human fibroblasts 1mmol/L. Unprogrammed DNA synthesis: human HeLa cells 50 μmol/L. Micronucleus test: hamster embryo 1μmol/L. Sister chromatid exchange: hamster ovary 100 μmol/L.
4. Carcinogenicity[17] IARC Carcinogenicity Comment: G2A, possible human carcinogen.
Ecological data
1. Ecotoxicity[18]
LC50: 6mg/L (96h) (fathead minnow , static); 4mg/L (96h) (zebrafish, static); 1.9mg/L (96h) (medaka)
EC50: 3.2mg/L (48h) (Daphnia)
p>
2. Biodegradability[19]
Aerobic biodegradation (h): 168 ~672
Anaerobic biodegradation (h): 672~2688
3. Non-biodegradability[20]
The half-life of photooxidation in air (h): 22~218
The half-life of primary hydrolysis (h): 15
Molecular structure data
1. Molar refractive index: 36.01
2. Molar volume (cm3/mol): 117.1
3. Isotonic specific volume (90.2K ): 282.5
4. Surface tension (dyne/cm): 33.8
5. Polarizability: 14.27
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 55.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It will be slowly hydrolyzed in hot water to form benzyl alcohol. Aromatic rings can undergo chlorination, nitration, sulfonation and other reactions. Chloromethyl can be hydrolyzed and oxidized to produce benzyl alcohol and benzoic acid.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidants, iron, iron salts, aluminum, water, alcohols
4. Conditions to avoid contact [23] Humid air, heat
5. Polymerization hazard[24] No polymerization
6. Decomposition products [25] Hydrogen chloride
Storage method
Storage Precautions[26] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 30°C and the relative humidity does not exceed 70%. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, metal powders, alcohols, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Toluene continuous photochlorination method Add dry iron-free toluene into an iron-free reactor and perform high-temperature chlorination under light. The chlorination product is then fractionated to obtain benzyl chloride, the by-product hydrogen chloride is absorbed with water to obtain hydrochloric acid, and the by-product α-dichloromethylbenzene can be hydrolyzed to produce benzaldehyde.
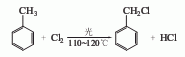
2. Toluene non-photothermal chlorine Chemical method: Toluene is first allowed to stand for more than 6 hours to remove iron, and then sent to the chlorination pot through a high-level tank. It is heated to boiling with steam and then stopped heating. Chlorine gas is introduced for chlorination. The benzyl chloride produced by the continuous reaction passes through the bottom. The overflow pipe enters the storage tank after cooling. Benzyl chloride is mainly produced by chlorination of toluene. At present, there are two main methods at home and abroad: photochlorination method and photothermal chlorination method. In terms of process operation, the two methods include intermittent operation and continuous operation. According to the reactor structure, continuous operation can be divided into two types: horizontal continuous operation and vertical continuous operation. Since the continuous photochlorination of toluene has the advantages of being easy to start in small batches and having a faster reaction speed than the photothermal chlorination method, many domestic manufacturers adopt this process. Generally, small horizontal series continuous reactor groups are used. Add dry iron-free toluene into a glass chlorination bottle, heat it to 110°C, and slowly boil the liquid. According to the mass ratio of chlorine gas to toluene is 1:2.5, introduce chlorine gas under fluorescent lamp irradiation, and proceed at 110-120°C. High temperature chlorination reaction to the end. Recover unreacted toluene by distillation, and collect the 160-180°C fraction to obtain crude benzyl chloride. Then carry out fractionation under normal pressure and collect the 176-182°C fraction to obtain the finished product of benzyl chloride.
Toluene photothermal chlorination generally uses large vertical continuous enamel reactors. In addition, benzyl chloride can be produced by reacting toluene with sulfur chloride in the presence of a catalyst peroxide, or by chloromethylating the benzene ring under the catalysis of zinc chloride.
3. Preparation method:
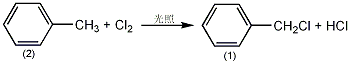
In a reaction bottle equipped with a thermometer, a reflux condenser (a calcium chloride drying sound is connected to the top, and the drying tube is connected to a hydrogen chloride and chlorine gas absorption device) and a ventilation tube, add 92g (1.0mol) of toluene (2) , a few grains of zeolite, heated in an oil bath until boiling. Use a 200W light bulb to illuminate ①, pass in chlorine gas dried with concentrated sulfuric acid (directly to the bottom of the reaction bottle), and continue to pass chlorine in a slightly boiling state until the temperature in the bottle rises to 156°C. , stop passing chlorine. Atmospheric pressure distillation, first recover unreacted toluene, and then collect the fraction between 165 and 185°C. Redistill and collect the fraction between 178 and 182°C. 90 g of benzyl chloride (1)① was obtained, with a yield of 70%. Note: ① Fluorescent lamp or quartz lamp can also be used for irradiation. This reaction is a free radical reaction. ② It can also be prepared by reacting methyl with sulfoxide chloride in the presence of amyl peroxide, or by reactions such as chloromethylation of benzene. [28]
Purpose
Used as dye intermediates and in the synthesis of tannins, spices, pharmaceuticals, etc. [27]
�Collect the fractions between 165 and 185°C. Redistill and collect the fraction between 178 and 182°C. 90 g of benzyl chloride (1)① was obtained, with a yield of 70%. Note: ① Fluorescent lamp or quartz lamp can also be used for irradiation. This reaction is a free radical reaction. ② It can also be prepared by reacting methyl with sulfoxide chloride in the presence of amyl peroxide, or by reactions such as chloromethylation of benzene. [28]
Purpose
Used as dye intermediates and in the synthesis of tannins, spices, pharmaceuticals, etc. [27]

 微信扫一扫打赏
微信扫一扫打赏

