Background and overview[1]
2-Fluorophene ether can be used as a pharmaceutical synthesis intermediate. If 2-fluorophenylethyl ether is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes contact, The eyelids should be separated, rinsed with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
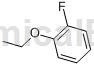
Preparation[1]
The preparation of 2-fluorophenylethyl ether is as follows:

The specific steps are as follows: Dissolve 2-fluorophenol (6.7g, 60mmol) in 66mL acetone, add ethyl iodide (6.3mL, 78mmol) and potassium carbonate (12.4g, 90mmol), and reflux the reaction in an oil bath 5 hours. Concentrate the reaction solution under reduced pressure, add 100 mL of ethyl acetate and 60 mL of water, separate the layers, extract the aqueous phase with ethyl acetate (30 mL × 2), combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title The product was 2-fluorophenylethyl ether (6.88g, red oil). MSm/z(ESI): 280.2[2M+1].
Apply[1]
2-Fluorophene ether can be used as a pharmaceutical synthesis intermediate. For example, prepare (5-bromo-2-chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanone:
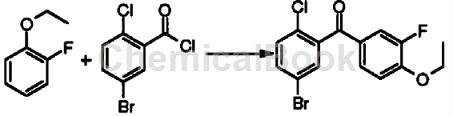
The specific steps are as follows: Dissolve 5-bromo-2-chloro-benzoyl chloride (12.4g, 48.8mmol) in 100mL dichloromethane, add 1-ethoxy-2-fluoro-benzene (6.84g, 48.8mmol), cool to 0°C, add aluminum trichloride (5.86g, 44mmol) in batches, and react for 16 hours. Add 20 mL of 2M hydrochloric acid solution dropwise to the reaction solution under an ice bath, separate the layers, extract the aqueous phase with 30 mL of methylene chloride, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title product (5-bromo -2-Chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanone (12.8 g, yellow solid). MSm/z(ESI): 358.9[M+1].
Main reference materials
[1] (CN104031098) Hypoglycemic drugs

 微信扫一扫打赏
微信扫一扫打赏

