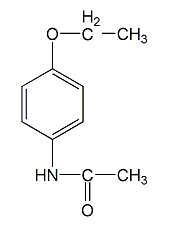
Structural formula
| Business number | 01CA |
|---|---|
| Molecular formula | C10H13NO2 |
| Molecular weight | 179.22 |
| label |
4′-Ethoxyacetanilide, N-(4-Ethoxyphenyl)acetamide, 1-Acetyl-p-phenetidin, N-(4-Ethoxyphenyl)acetamide, Phenacetin, CH3CONHC6H4OC2H5 |
Numbering system
CAS number:62-44-2
MDL number:MFCD00009094
EINECS number:200-533-0
RTECS number:AM4375000
BRN number:1869238
PubChem number:24890660
Physical property data
1. Character:White shiny scaly crystals or white crystalline powder. Odorless, slightly bitter taste.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):133-136 (lit.)
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 4mmHg):132
7. Refractive index:1.571
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
1)It is derived from acetylation of p-aminophenylene ether. The mixture of benzene, acetic anhydride and p-aminophenylene ether is heated to azeotrope in an oil bath4h , after the reaction is completed, the reactant is cooled, and phenadine is precipitated, which is obtained by filtering, washing with cold benzene, and drying. The yield is the theoretical amount86%.
(2)Derived from the reaction of acetaminophen and sodium ethoxide: first, the acetamide group Add phenol to sodium ethoxide, then slowly add ethyl iodide, and heat to reflux1h, cooling, Filter, dissolve the crude product in ethanol, filter, dilute the filtrate with hot water, and then cool it, Filter and dry to obtain the finished product, the yield is 80% of the theoretical amount80%. The first method can also use acetic acid instead of benzene as the solvent. The process is as follows. 40%Heat the dilute acetic acid to boiling, add p-aminophenylene ether, evaporate the water, and wait The temperature rises to 150℃add glacial acetic acid and reflux1h, continue steaming until the temperature rises to 150℃Above, take a sample to measure free aminophenylene ether, add acetic anhydride according to the amount of residual aminophenylene ether, and reflux reaction0.5h, check the end point, reduce the pressure after passing the test, and recover acetic acid until the amino content is within 0.046The following, containing acid0.2%Following, pour the reaction fluid into the hot refining mother liquor, stir and cool down to 40 ℃, filter to obtain crude phenacetin. To refine, add boiling water or refined mother liquor to crude phenacetin, heat and stir to dissolve, filter, and adjust the filtrate with acid to pH=4.5-4.7, add activated carbon and sodium thiosulfate, stir to decolorize, filter, cool and crystallize the filtrate, filter out, and air dry to obtain phenacetin.
Purpose
For acetaminophen ether Used as raw materials for organic synthesis and pharmaceutical intermediates.
US style=”FONT-SIZE: 9pt; FONT-FAMILY: Times New Roman”>pH=4.5-4.7, add activated carbon and sodium thiosulfate, stir to decolorize, filter, cool the filtrate to crystallize, filter, air dry, and obtain phenadine Sitting.
Purpose
For acetaminophen ether Used as raw materials for organic synthesis and pharmaceutical intermediates.

 微信扫一扫打赏
微信扫一扫打赏

