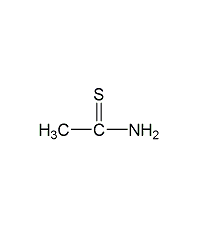
Structural formula
| Business number | 01CF |
|---|---|
| Molecular formula | CH3CSNH2 |
| Molecular weight | 75.13 |
| label |
Ethanethioamide, Acetothioamide, Ethylthionamide;, Qualitative analysis |
Numbering system
CAS number:62-55-5
MDL number:MFCD00008070
EINECS number:200-541-4
RTECS number:AC8925000
BRN number:506006
PubChem number:24889097
Physical property data
1. Properties: colorless or white crystal. New products sometimes have a mercaptan smell and slightly absorb moisture.
2. Density (g/mL, 25/4℃): 1.37
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 108-112(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º ): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Solubility in water at 25°C 16.3g/100ml, ethanol 26.4g/100ml. Very slightly soluble in benzene and ether. Its aqueous solution is quite stable at room temperature or 50-60°C, but when hydrogen ions are present, it quickly generates hydrogen sulfide and decomposes
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 22.13
2. Molar volume (cm3/mol): 70.2
3. Isotonic specific volume (90.2K): 187.3
4. Surface tension (dyne/cm): 50.6
5. Polarizability (10-24cm3): 8.77
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): -0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. TopologyPolar surface area of �� ions: 58.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 33
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
The aqueous solution is quite stable at room temperature or 50-60°C, but when hydrogen ions are present, hydrogen sulfide is quickly generated and decomposes. New products sometimes have a mercaptan smell and slightly absorb moisture.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1. Thioacetamide can be prepared by reacting acetonitrile with hydrogen sulfide, or by reacting acetamide with phosphorus pentasulfide.
2. First heat the solvent benzene to 70°C, then add industrial product acetamide and stir until completely dissolved. Then slowly add ground industrial product phosphorus pentasulfide under rapid stirring (the amount of acetamide is 5 times the calculated amount). The reaction solution continued to be heated until it changed from green to yellow. Stop stirring, carefully separate the yellow solution, cool and crystallize, let stand for 2 hours, filter, and dry the solid in the air. Distill the filtrate to remove 2/3 of the benzene, cool and crystallize, filter and combine with the solid obtained above to obtain the finished product. The resulting filtrate can also recover some products.
The process reaction formula is:
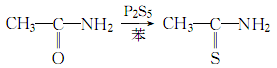
Purpose
1. Used in the production of catalysts, stabilizers, polymerization inhibitors, electroplating additives, photographic chemicals, pesticides, dyeing auxiliaries and mineral processing agents, etc. It is also used as vulcanizing agent, cross-linking agent, rubber additive and pharmaceutical raw material for polymers.
2. Used for qualitative analysis, replacing hydrogen sulfide gas as a grouping reagent.

 微信扫一扫打赏
微信扫一扫打赏

