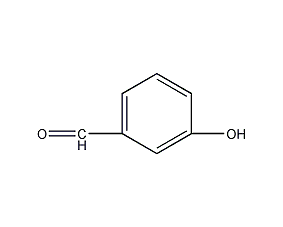
Structural formula
| Business number | 02JS |
|---|---|
| Molecular formula | C7H6O2 |
| Molecular weight | 122.12 |
| label |
3-Hydroxybenzaldehyde, 3-Hydroxybenzaldehyde, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:100-83-4
MDL number:MFCD00003368
EINECS number:202-892-9
RTECS number:CU6450000
BRN number:507099
PubChem number:24895491
Physical property data
1. Properties: colorless or light yellow crystalline solid.
2. Relative density (25℃, 4℃): 1.1011150.4
3. Relative density (d1304) : 1.1179
4. Melting point (ºC): 108
5. Boiling point (ºC, normal pressure): 240, 191ºC (6665pa)
p>
6. Boiling point (ºC, 61KPa): 191
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20ºC ): Undetermined
12. Saturated vapor pressure (kPa, 20ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined Determined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, soluble in hot water, ethanol, acetone, ether and benzene.
Toxicological data
Sister chromatid exchange test: human lymphocytes, 1mmol/L;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 34.88
2. Molar volume (cm3/mol): 99.5
3. Isotonic specific volume (90.2K ): 267.3
4. Surface tension (dyne/cm): 52.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides and air.
2.The gas forms an explosive mixture with air. Combustion produces irritating, corrosive and/or toxic gases. Harmful by inhalation, ingestion and skin contact. Protective glasses, protective clothing and protective gloves should be worn.
3. Found in tobacco leaves.
4. Irritating.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. should be kept away from oxidizer, do not store together. Keep sealed. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. Packed in cardboard drums, net weight 25kg per drum.
Synthesis method
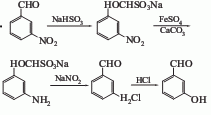
Using m-nitrobenzaldehyde as raw material, there are two methods for preparing m-hydroxybenzaldehyde. 1. Obtained from m-nitroformaldehyde through addition, reduction, diazotization and hydrolysis. Dissolve sodium bisulfite in water, add m-nitrobenzaldehyde, stir and dissolve at 50°C to obtain an adduct. The adduct is in the presence of calcium carbonate, heated to reflux with ferrous sulfate for 3 hours, and reduced to an amino compound. Cool the reducing liquid to below 15°C, add sodium nitrite solution dropwise for diazotization, and then hydrolyze at 100-110°C to obtain m-hydroxybenzaldehyde. After decolorization with activated carbon and recrystallization with water, the finished product is obtained.
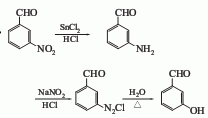
2. From m-nitrobenzene Formaldehyde is obtained by reduction of stannous chloride, diazotization and hydrolysis. Prepare a solution of powdered stannous chloride dihydrate and hydrochloric acid, cool to 5°C, and add m-nitrobenzaldehyde. After slowly raising the temperature to 25-30°C, the temperature rises rapidly. When it reaches 100°C, cool and stir vigorously to obtain an orange paste. Add concentrated hydrochloric acid to form a suspension, slowly add sodium nitrite aqueous solution while stirring, and control the temperature at 4-5°C. After adding, stir for 1 hour. Cool and crystallize, filter out the diazonium salt, add it to boiling water for hydrolysis, and use activated carbon to decolorize. Filter while hot, cool and crystallize to obtain the finished product.
Purpose
Meta-hydroxybenzaldehyde is used as an intermediate for fine chemicals such as pharmaceuticals, dyes, fungicides, and photographic emulsifiers.

 微信扫一扫打赏
微信扫一扫打赏

