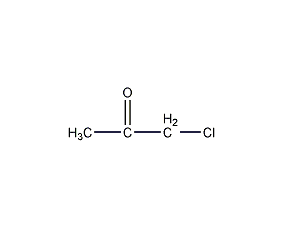
Structural formula
| Business number | 01NZ |
|---|---|
| Molecular formula | C3H5ClO |
| Molecular weight | 92.52 |
| label |
None yet |
Numbering system
CAS number:78-95-5
MDL number:MFCD00000936
EINECS number:201-161-1
RTECS number:UC0700000
BRN number:605369
PubChem number:24846855
Physical property data
1. Properties: Colorless liquid with a strong irritating odor. Tear-jerking.
2. Density (g/mL, 25/4℃): 1.1260
3. Relative density (20℃, 4℃): 1.14819.5
4. Melting point (ºC): -44.5
5. Boiling point (ºC, normal pressure): 119.5
6. Boiling point (ºC, 5.2kPa) : 61 (ºC, 6.67kpa)
7. Refractive index: 1.4350
8. Flash point (ºC): 27 (open cup)
9. Refractive index at room temperature (n20): 1.43419.5
10. Refractive index at room temperature (n25): 1.4300
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion ( KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Miscible with ethanol, ether and chloroform, and soluble in 10 times the weight of water.
Toxicological data
1. Acute toxicity
Human oral LCL0: 605 ppm/10M
Rat LD50: 100mg/kg; rat inhalation LC50: 262ppm/1H
Rat abdominal cavity LD50: 80 mg/kg
Mouse abdominal cavity LD50: 127mg/kg;
Mouse abdominal cavity LD50: 92 mg/kg
Rabbit skin LD50: 141 mg/kg
Pig skin LD50: 100 uL/kg
2. Other multi-dose toxicity data
Rat caliber TCLO: 650 mg/kg/17D-I
3. Teratogenicity
Salmonella: 6mg/L
Drosophila: 100 pph/6M
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 20.82
2. Molar volume (cm3/mol): 86.5
3. Isotonic specific volume (90.2K ): 195.0
4. Surface tension (dyne/cm): 25.7
5. Polarizability (10-24cm3): 8.25
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): 0.6
2. Number of hydrogen bond donors: 0
3. Hydrogen bondsNumber of receptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecular polarity Surface area 17.1
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 42.2
10 .The number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It can evaporate with water vapor, darken and become resinous under light. Add 0.1% water or 1% calcium carbonate as a stabilizer.
2.It is highly irritating to biological tissues. It decomposes under the action of sunlight to produce a highly tear-inducing gas. The gas is 0.018mg /L has a tear-inducing effect. Humans can endure less than 1 minute in an atmosphere of 0.11mg/L. During production, the equipment should be sealed and operators should wear protective gear, paying special attention to eye protection.
Storage method
This product should be sealed and stored in a cool, dark place. Packed in 500mL glass bottles and external wooden boxes, handle with care. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Acetone chlorination method: Stir acetone and calcium carbonate to form a slurry, heat to reflux, stop heating and add chlorine gas. After about 3-4 hours, add water to dissolve the generated calcium chloride, and the reaction solution is divided into two layers. The oil layer is collected and then refined to obtain the finished product.
![]()
2. Non-chlorine chlorination of acetone The method uses acetone as raw material and is obtained by chlorinating the chlorine gas produced by the reaction and decomposition of oxidants such as potassium chlorate and hydrogen chloride.
![]()
3. Chlorinated cyanuric acid The acid method uses trichlorocyanuric acid as the halogenating agent, sulfuric acid aqueous solution as the catalyst, and performs a chlorination reaction on acetone. This process was developed abroad in the early 1990s. Its synthesis method is simple and its product content is high.
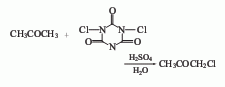
Purpose
Used as an organic synthesis intermediate in the production of dyes, pesticides, medicines, spices, antioxidants, desiccants, vinyl photosensitive resins, color film coupling agents, etc.

 微信扫一扫打赏
微信扫一扫打赏

