Background and overview[1]
Phenyl trifluoromethanesulfonate, also called phenyl triflate, can be used in organic synthesis and can be obtained by acylation of phenol.
Preparation[1-3]
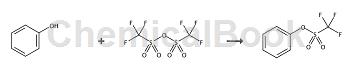
Phenyl triflate: Triflate (10g, 35mmol) was added dropwise to a solution of phenol (3.16g, 35mmol) in pyridine (60mL) at 0°C and argon. The mixture was stirred for 24 hours, keeping the temperature below 5 °C, then poured into diethyl ether (100 mL) and washed with water (3 x 60 mL). The organic portion was dried (MgSO4) and evaporated under reduced pressure to give the triflate (0.93g, 77%); 1H NMR (CDCl 3, 400 MHz): δ 7.24-7.26 (2H, m), 7.35-7.36 (1H, m), 7.39-7.43 (2H, m). 13C NMR (CDCl 3, 100 MHz): δ 114.1-126.6 (CF3, q, J = 310 Hz), 121.3, 128.4, 130.3, 149.7.
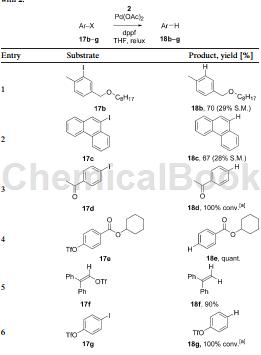
Method 2, phenyl triflate and 18g in the picture below, can be prepared by 4-iodo phenyl triflate under the catalysis of palladium acetate.
Apply[4]
Can be used to prepare (S)-tert-butyl 2-phenylpropionate.
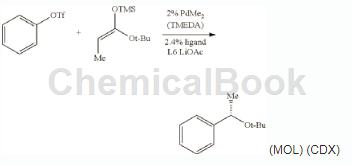
In an argon-filled glove box, add PdMe2 (TMEDA) (2.5 mg, 0.010 mmol), (R)-2-(dicyclohexylphosphine) to a dry 4 mL vial base)-2′-(2-naphthylmethoxy)-5, 5′, 6,6′, 7,7′, 8,8′-octahydro-1,1′-binaphthyl L6 (7.4 mg , 0.012 mmol) and 1.0 mL of dry α, α, α-trifluorotoluene. After stirring at 25°C for 30 minutes, add anhydrous LiOAc (66 mg, 1.0 mmol), phenyl triflate (113 mg, 0.50 mmol), (1E)-1-tert-butoxy-1-(trifluoromethanesulfonate) in sequence. Methylsiloxypropylene (152 mg, 0.75 mmol) and GC standard n-dodecane (50 μL). Cap the vial tightly and stir the mixture in a 50°C (internal temperature) heating block.��Hot. After complete consumption of phenyl triflate within 18 h at 50 °C (monitored by OC and TLC), the reaction mixture was cooled to 25 °C and filtered through a pad of silica gel with ether washes (20 mL). The filtrate was concentrated and the residue was purified by flash silica gel chromatography using ethyl acetate/hexane (1:40) as eluent to give the title compound as a colorless oil (96 mg, 93% yield).
Main reference materials
[1] Sabin, Margaret V . Synthetic studies on two silicon assisted annulation reactions.[J]. Aiche Journal, 1994, 55(55):594-611.
[2] Zhu S , Wang C , Chen L , et al. Modular Approach for Synthesis of Vicinal Diamines Containing Axial Chiral 1,1′-Binaphthyl from 1,2-Diaminoethane by Pd-Catalyzed N-Arylation Reactions[J ]. Organic Letters, 2011, 13(5):1146-1149.
[3] Chu Q , Brahmi M M , Solovyev A , et al. Ionic and Organometallic Reductions with N-Heterocyclic Carbene Boranes[J]. Chemistry, 2009, 15(47):12937-12940.
[4] (US20150166586) Chiral phosphines for palladium-catalyzed asymmetric α-arylation of ester enolates to produce tertiary stereocenters in high enantioselectivity

 微信扫一扫打赏
微信扫一扫打赏

