Background and overview[1]
5-Phenyl-o-anisidine, also called 3-amino-4-methoxybiphenyl, can be prepared from p-hydroxybiphenyl through a three-step reaction.
Preparation[1]
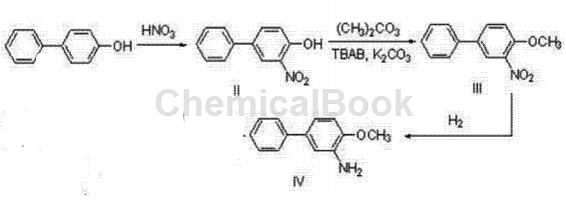
Preparation of compound biphenylhydrazine ester (I)
In a 250ml three-necked flask, dissolve 5g (29.4mmol) of p-hydroxybiphenyl in 25ml of methyl tert-butyl ether (MTBE), mix and stir for 5 minutes, and control the temperature at 30°C. Take 4ml (58.8mmol, 2eq.) of nitric acid and 10ml of MTBE and pour it into the dropping funnel. Complete the addition within 1 hour and continue stirring for 3 hours. TLC detection, the reaction is completed. Pour the system into 300 ml of ice water and stir for 5 minutes. At this time, there is no deep orange liquid on the surface of the liquid surface, and a large amount of solid precipitates. Filter and dry to obtain 5.9 g of yellow solid 3-nitro-4-hydroxybiphenyl (II), which is collected. The rate is 93%.
Put 5g (23.2mmol) of the obtained 3-nitro-4-hydroxybiphenyl solid with 40ml dimethyl carbonate, 8.2g (1.1eq.) tetrabutylammonium bromide (TBAB) and 3.2g (1eq. .) Potassium carbonate, stir and mix, raise the temperature to 100°C and reflux. TLC detection was performed after 48 hours, and the reaction was completed. Cool the system to room temperature, add HCl solution dropwise to the system, and adjust to pH 4-6. At this time, no bubbles emerge from the system. The system was poured into 200 ml of ice water, a large amount of solid precipitated, filtered with suction, and dried to obtain 5.253 g of yellow-brown solid 3-nitro-4-methoxybiphenyl (III), with a yield of 98.7%.
Add 5g of the obtained 3-nitro-4-methoxybiphenyl (III) into the reaction bottle, add 20ml of methanol, use 5% Raney nickel as the catalyst, and react for 3 hours at 60°C and 1.5MPa. After TLC detection showed that the reaction was completed, methanol was removed by vacuum rotary evaporation to obtain 4.0g of the desired product compound 3-amino-4-methoxybiphenyl (IV), with a yield of 93%.
Apply[1]
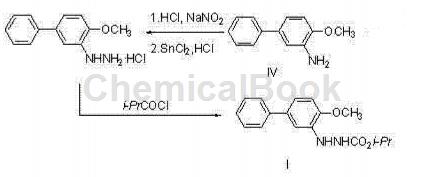
Can be used to prepare biphenyl hydrazine ester, as shown in the picture above. The chemical name of bifenazate is N’-(4-methoxybiphenyl-3-)hydrazinecarboxylic acid isopropyl ester, which is a new type of hydrazine ester compound. It is a specific acaricide that is active during the active phase of spider mites and can also control the egg stage of pests such as two-spotted spider mites. Used on cotton, hops and some fruit trees to effectively control mites at all growth stages. This drug can provide rapid killing and long-residue selective control effects (harmless to beneficial mites and beneficial insects). It has been registered for use in various states in the United States, and is currently not produced in China.
Main reference materials
[1] CN201110212882.4 A kind of synthesis method of biphenyl hydrazine ester

 微信扫一扫打赏
微信扫一扫打赏

