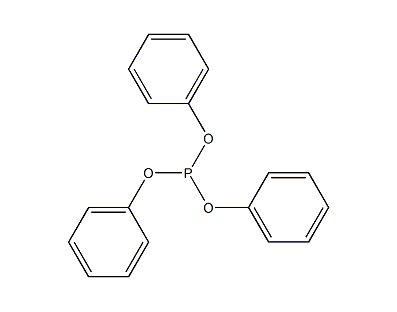
Structural formula
| Business number | 02K1 |
|---|---|
| Molecular formula | C18H15O3P |
| Molecular weight | 310.28 |
| label |
TTP, TTP, flame retardant; stabilizer |
Numbering system
CAS number:101-02-0
MDL number:MFCD00003032
EINECS number:202-908-4
RTECS number:TH1575000
BRN number:1079456
PubChem number:24900533
Physical property data
1. Properties: Colorless to light yellow monoclinic crystals below room temperature. It is a light yellow transparent liquid with a phenol smell when it is above room temperature
2. Melting point (ºC): 22~23
3. Boiling point (ºC, 0.1Mpa): 360
4. Boiling point (ºC, 1.3kpa): 220
5. Flash point (ºC, open cup): 218.3
6. Refractive index: 1.589
7. Solubility: Insoluble in water, soluble in organic solvents such as alcohol, ether, benzene and acetone.
Toxicological data
1. Irritation: Human transdermal: 125mg/48 hours, severe irritation. Rabbit transdermal: 500mg, severe irritation.
2. Acute toxicity: Rat oral LD5O: 1600-3200mg/kg; mouse abdominal LD5O: 50-100mg/kg
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 5.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 247
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Decomposes on contact with moisture. Avoid contact with strong oxidants, strong acids, and strong alkali.
Storage method
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Phosphorus oxychloride direct method (also known as thermal method) phenol uses pyridine and anhydrous benzene as solvents, slowly adds phosphorus oxychloride at a temperature not exceeding 10°C, and then at reflux temperature, Reaction takes 3 to 4 hours. After cooling to room temperature, the reactant was washed with water to recover pyridine, centrifuged and dehydrated, then dehydrated with dry sodium sulfate, and filtered to remove sodium sulfate. Finally, normal pressure distillation is used to recoverBenzene is distilled under reduced pressure, and the fractions at 243-245°C (1.47kPa) are collected. After cooling, crystallization and crushing, the finished product is obtained.
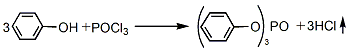
2. Phosphorus trichloride indirect method (also known as cold method): After phenol is melted, phosphorus trichloride is added dropwise at 25°C with stirring to generate triphenyl phosphite; then the temperature is raised to 70°C and chlorine gas is introduced to generate dichlorophosphoric acid Triphenyl ester; then add water for hydrolysis at 50℃ to generate triphenyl phosphate. Finally, the hydrolyzate is neutralized, washed, evaporated and distilled under reduced pressure with 5% soda ash aqueous solution. The fractions at 243~245℃ (1.47kPa) are collected, cooled, crystallized, crushed and packaged to become the finished product.
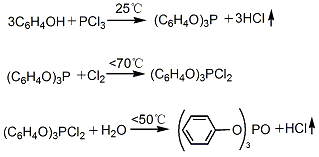
Purpose
1. Used as a stabilizer for synthetic rubber and resin, an antioxidant for polyvinyl chloride plastics, and a raw material for alkyd resin and pesticide intermediates. As a flame retardant plasticizer for cellulose resins, vinyl resins, natural rubber and synthetic rubber. This product has low volatility, high flame retardant efficiency, and excellent mechanical property retention, transparency, softness and toughness. It is mainly used as a flame retardant plasticizer for nitrocellulose, cellulose acetate films (such as film bases) and film plastics. It can also be used as a non-flammable substitute for camphor in viscose fibers.
2. Used as an anti-corrosion agent for polymers such as polyethylene, polypropylene, polyvinyl chloride, ABS resin, epoxy resin, synthetic rubber and polyester. Oxygen stabilizer. Used together with halogen flame retardants, it can exert its flame retardant and antioxidant effects, and has light stabilization function. It is also widely used as a chelating agent for polyvinyl chloride. When a stabilizer based on metal soap is used, this product can reduce the harm of metal chlorides, maintain the transparency of the product, and inhibit changes in color. It can also be used to synthesize alkyd resin, polyester, alkyl phosphite, etc.

 微信扫一扫打赏
微信扫一扫打赏

