Background and overview[1]
4-Aldehyde benzocyclobutene is an organic intermediate that can be prepared from 4-bromobenzocyclobutene.
Preparation[1]
Add 50 mL of anhydrous tetrahydrofuran (THF), Mg chips (2.88 g, 120 mmol) and 1,2-dibromoethane (4 drops) into a 500 mL flask. The reaction mixture was then heated at reflux for 15 minutes. A solution of 4-bromobenzocyclobutene (1.1, 20 g, 109 mmol) in 25 mL of anhydrous THF was added dropwise to form a Grignard reagent. After the addition was completed and the dropping funnel was rinsed with 25 mL of anhydrous THF, the reaction mixture was heated under reflux for another 45 minutes to obtain a green-brown solution. The reaction mixture was then cooled to 0°C. Dimethylformamide (DMF) (15 mL, 210 mmol) was added dropwise to the solution, and the reaction mixture was heated to reflux for 15 minutes. The reaction mixture was poured into 150 g of ice, acidified and neutralized with saturated NaHCO3 solution. The crude product was extracted with ethyl acetate, the organic phase was filtered through Celite, and the solvent was evaporated to obtain the crude product. The product was purified by column chromatography using 10% ether/hexane as the elution solvent to obtain the corresponding aldehyde (1.2, 12 g, 82%) as a colorless liquid. 1 H NMR (300MHz, CDCl3): d 9.9 (s, 1H), 7.65 (dd, 1H), 7.50 (s, 1H), 7.14 (dd, 1H), 3.15(s, 4H).
Apply[1]
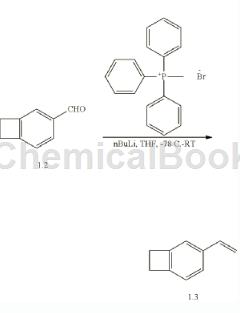
Can be used to prepare 4-vinylbenzocyclobutene. Add methyltriphenylphosphonium bromide 1.1 (48.6g, 136.2mmol) and 220mL anhydrous THF to a 500mL round-bottom flask, cool the solution to -78°C, and add n-BuLi (2.5M hexane solution) dropwise , 52.8 mL, 132 mmol), and the reaction mixture was warmed to room temperature. The yellow-orange solution was cooled to -78 °C and aldehyde 1.2 (14.32 g, 108.4 mmol) diluted in anhydrous THF (70 mL) was slowly added. The mixture was warmed to room temperature and stirring continued for 2 hours. The reaction was treated with saturated NH4Cl and saturated NaHCO3 solutions in sequence. The crude product was filtered through diatomaceous earth, washed with ether/hexane (1:1), and evaporated to Dry (without heating) to obtain crude product. Further purification by column chromatography using 5% diethyl ether/hexane as elution solvent followed by vacuum distillation (75°C, 1.0 mm) afforded pure 4-vinylbenzocyclobutene 1.3 as a colorless liquid (11 g, 78%). 1H NMR (300MHz, CDCl3): δ7.26 (d, 1H), 7.20 (s, 1H), 7.04 (d, 1H), 6.74 (dd , 1H), 5.70 (d, 1H), 5.20 (d, 1H), 3.19 (s, 4H).
Main reference materials
[1] (US20150132921) Gap-fill methods

 微信扫一扫打赏
微信扫一扫打赏

