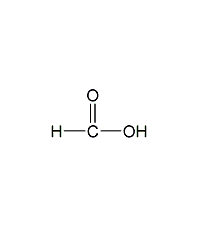
Structural formula
| Business number | 01D7 |
|---|---|
| Molecular formula | CH2O2 |
| Molecular weight | 46.03 |
| label |
formic acid, anhydrous formic acid, Formic acid solution, Methanoic acid, Tanning softener for leather industry, Mordant for printing and dyeing industry, natural rubber coagulant, disinfectant, preservative |
Numbering system
CAS number:64-18-6
MDL number:MFCD00003297
EINECS number:200-001-8
RTECS number:LQ4900000
BRN number:1209246
PubChem number:24873243
Physical property data
1. Characteristics: Colorless and transparent fuming liquid with a strong pungent sour smell. [1]
2. pH value: 2.2 (1% solution) [2]
3. Melting point (℃ ): 8.4[3]
4. Boiling point (℃): 100.8[4]
5. Relative density ( Water=1): 1.23[5]
6. Relative vapor density (air=1): 1.59[6]
7. Saturated vapor pressure (kPa): 5.33 (24℃)[7]
8. Heat of combustion (kJ/mol): -254.4[8 ]
9. Critical temperature (℃): 315[9]
10. Critical pressure (MPa): 8.63 [10]
11. Octanol/water partition coefficient: -0.54[11]
12. Flash point (℃): 68.9 (OC); 69 (CC) [12]
13. Ignition temperature (℃): 480[13]
14. Explosion upper limit (%): 57.0[14]
15. Explosion lower limit (%): 12.0[15]
16. Solubility: miscible with water, insoluble in hydrocarbons, miscible in ethanol and ether, and soluble in benzene. [16]
17. Refractive index (n20ºC): 1.37140
18. Refractive index (n25ºC): 1.3693
19 . Viscosity (mPa·s, 25ºC): 1.966
20. Viscosity (mPa·s, 30ºC): 1.443
21. Heat of evaporation (KJ/mol, 25ºC): 19.90
22. Heat of evaporation (KJ/mol, b.p.): 23.19
23. Heat of fusion (KJ/mol): 12.69
24. Heat of generation ( KJ/mol, 25ºC, liquid): -425.04
25. Heat of combustion (KJ/mol, 25ºC, liquid): 254.81
26. Specific heat capacity (KJ/(kg·K ), 26.68ºC, constant pressure): 2.15
27. Thermal conductivity (W/(m·K), 15ºC): 13.9148
28. Thermal conductivity (W/ (m·K), 30ºC): 13.8456
29. Thermal conductivity (W/(m·K), 60ºC): 13.7744
30. Thermal conductivity (W/ (m·K), 90ºC): 13.6033
31. Relative density (20℃, 4℃): 1.2201
32. Solubility parameter (J·cm– 3)0.5: 21.442
33.van der Waals area (cm2·mol-1 ): 3.634×109
34. van der Waals volume (cm3·mol-1): 22.740
35. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -254.2
36. Liquid phase standard claimed heat (enthalpy) )( kJ·mol-1): -425.1
37. Liquid phase standard entropy (J·mol-1</suIt has a wide range of uses, such as: ethyl formate – peach, berry and other fruit flavors; isoamyl formate – fruit flavor, leather flavor; hexyl formate – apple flavor; heptyl formate – apricot, plum , peach and other fruit flavors; n-decyl formate – used for orange blossom essence and iris oil; benzyl formate – used for jasmine and other floral fragrances and soap flavors; cinnamyl formate – used for jasmine, moonflower and other flavors; citronella formate Esters – used in rose, osmanthus, wild lily and other flavors; syringyl formate – used in carnation flavors, etc.; geranyl formate – used in rose, orange blossom, geranium and other flavors; linalyl formate – lavender , bergamot and other flavors; menthyl formate – cosmetic flavor, spray flavor; phenethyl formate – white rose, orchid, chrysanthemum and other flavors; thyme formate – cosmetic flavor.
2. Used as analytical reagents, such as reducing agents to prepare buffer solutions. It is also used for pure carbon monoxide preparation and pesticide preparation.
3. Sterilization and mildew prevention in pulp manufacturing.
4. It can also produce leather softeners, rubber coagulants, printing and dyeing mordants, fiber and paper dyes and treatment agents, plasticizers and animal feed additives, etc. It can also be used as a preservative in cosmetics. The maximum allowable content in cosmetics is 0.5% (acid).
5. Formic acid and its aqueous solution can dissolve many metals, metal oxides, hydroxides and salts. The generated formate can be dissolved in water and can therefore be used as a chemical cleaning agent. Formic acid does not contain chloride ions and can be used for cleaning equipment containing stainless steel materials.
6. Used in the preparation of chemicals, rubber coagulants, textiles, printing and dyeing, electroplating, etc. [28]

 微信扫一扫打赏
微信扫一扫打赏

