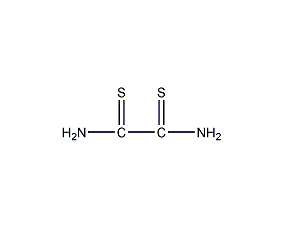
Structural formula
| Business number | 01PV |
|---|---|
| Molecular formula | C2H4N2S2 |
| Molecular weight | 120.20 |
| label |
dithiooxalamide, red acid, Dithiooxalic diamide, rubeanic acid, Photometric determination of metals |
Numbering system
CAS number:79-40-3
MDL number:MFCD00004941
EINECS number:201-203-9
RTECS number:RP1575000
BRN number:605577
PubChem number:24867415
Physical property data
1. Character: red crystal or crystalline powder
2. Density (g/mL, 25/4℃): Uncertain)
3. Relative vapor density ( g/mL, air=1): Uncertain
4. Melting point (ºC): 41℃, decomposes at 200℃.
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition Combustion temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18 . Lower explosion limit (%, V/V): Uncertain 19. Solubility: Soluble in alcohol and concentrated sulfuric acid and turns red. Slightly soluble in water, insoluble in ether
Toxicological data
1. Acute toxicity
Rat caliber LD50: 500mg/kg; mouse caliber LC50: 350mg/kg;
Rat abdominal LD50: 100mg/kg; Rat intravenous LD50: 56mg/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 32.96
2. Molar volume (cm3/mol): 78.8
3. Isotonic specific volume (90.2K ): 262.8
4. Surface tension (dyne/cm): 123.3
5. Polarizability (10-24cm3): 13.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Rotatable ChemistryNumber of bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 116
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 75.5
10. Number of isotope atoms: 0
11. Determine Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
In ammonia solution with Co2+, Ni2+ Pd2+ , Cu2+, Fe3+ and others form water-insoluble colored complexes.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
1. Add concentrated ammonia water to concentrated copper sulfate solution while stirring until the precipitated copper hydroxide is redissolved. After cooling, slowly add potassium cyanide solution with constant stirring until the blue color disappears. Then hydrogen sulfide gas produced by the reaction of sodium sulfide and hydrochloric acid is passed into the reaction liquid. Control hydrogen sulfide: cyanide gas = 3: 1, stir evenly, cool completely, let it stand, and filter. The obtained crystals are washed with cold water and recrystallized with ethanol to obtain the finished product
Dithiooxamide. The process reaction formula is as follows:
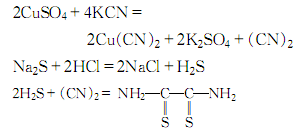
Purpose
1. Precipitating agent for determination of copper, cobalt and nickel. Drop test reagents for bismuth, cobalt, copper, nickel, platinum and palladium. Stabilizer for vitamin C solutions.

 微信扫一扫打赏
微信扫一扫打赏

