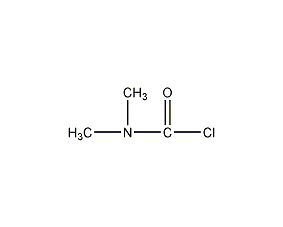
Structural formula
| Business number | 01PZ |
|---|---|
| Molecular formula | C3H6ClNO |
| Molecular weight | 107.54 |
| label |
N,N-dimethylcarbamoyl chloride, Chloroformyldimethylamine, Chloroformic acid dimethyl amide, (CH3)2NCOCl, Multifunctional solvents, aliphatic compounds |
Numbering system
CAS number:79-44-7
MDL number:MFCD00000635
EINECS number:201-208-6
RTECS number:FD4200000
BRN number:878197
PubChem number:24893469
Physical property data
1. Properties: colorless or light yellow to light brown irritating oily liquid
2. Density (g/mL, 20/4℃): 1.1678
3. Melting point (ºC): -33
4. Boiling point (ºC, normal pressure): 167
5. Refractive index (20ºC): 1.4540
6. Flash Point (ºC): 68
7. Solubility: Soluble in organic solvents such as ether, carbon disulfide and benzene. Not miscible with water.
Toxicological data
Corrosive to skin, eyes and mucous membranes.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 24.54
2. Molar volume (cm3/mol): 93.8
3. Isotonic specific volume (90.2K ): 220.7
4. Surface tension (dyne/cm): 30.6
5. Polarizability (10-24cm3): 9.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 20.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 61.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Reacts with water to release hydrogen chloride smoke. Combustible. There is a danger of burning when exposed to high heat, flame or contact with oxidants. Tear-jerking agent.
2.This product may burn when exposed to high heat, fire or oxidants. Tear-jerking agent. Corrosive to skin, eyes and mucous membranes. Can produce toxic and corrosive fumes when in contact with water or water vapor. If your eyes are irritated, rinse with water if it splashes into your eyes.Severe cases must seek medical treatment. If the skin comes into contact, rinse with water first, and then wash thoroughly with soap. Those who inhale the vapor should stay away from the contaminated area, rest and keep warm. If swallowed, rinse your mouth immediately and be sent to the hospital for treatment.
Storage method
1. This product should be sealed and stored in a cool place.
2. This product is packed in a plastic bag lined with an iron drum outside.
Synthesis method
Prepared from the reaction of dimethylamine and phosgene, the reaction formula is as follows:

Add toluene into the reaction pot, and introduce phosgene under cooling conditions to absorb it to the calculated amount. In another pot, gaseous dimethylamine is generated from the dimethylamine aqueous solution, and after drying, it is passed into the phosgene toluene solution for reaction. After the reaction is completed, filter to remove the hydrochloride, distill under reduced pressure, and collect 75 (3.33kPa) fractions, which is dimethylcarbamoyl chloride, with a yield of 55%.
In addition, dimethylcarbamoyl chloride can also be prepared by reacting N,N-dimethylformamide with phosphorus trichloride and then with thionyl chloride. Or use Pd, PdCl2 or RhCl3 as catalyst and obtain it from the direct reaction of dimethylammonium chloride and carbon monoxide.
Purpose
Used for the synthesis of medicines, pesticides, dyes, plastics, synthetic materials and other fine organic chemical products, such as for the production of neostigmine bromide, neostigmine methyl sulfate, pyridostigmine, carbamic acid Ester pesticides, etc. Also used as solvent.

 微信扫一扫打赏
微信扫一扫打赏

