Background and overview[1]
2-Nitrophenyl silk cyanate can be used as a pharmaceutical synthesis intermediate. If 2-nitrophenyl silk cyanate is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
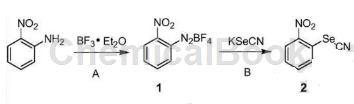
Step A: Add a solution of 2-nitroaniline (15.0g, 108mmol) in dichloromethane (150mL) dropwise to boron trifluoride ether (23.1g, 163mmol) at -10~-15°C. Stir for 15 minutes, then add a solution of isoamyl nitrite (15.26 g, 130 mmol) in dichloromethane (75 mL) dropwise at this temperature. After the addition, continue stirring for 30 minutes, and then stir at -10~0°C for 30 minutes. Add cold petroleum ether (250 mL) dropwise to the reaction system, filter, and wash the filter cake with cold methyl tert-butyl ether (MTBE) (40 mL) to obtain 2-nitro-phenyl-fluoroborate diazonium salt (1) (18.7g). The yield is 73.1%.
Step B: In an ice-water bath, a solution of potassium selenocyanate (8.0g, 55.5mmol) in water (80mL) was added dropwise to a mixture of compound 1 (13.0g, 54.9mmol) and water (300mL). When finished, continue stirring for 30 minutes. Filter, wash the filter cake with a small amount of water, and dry it under vacuum at 60°C to obtain 2-nitrophenyl silk cyanate (2) (11.2g). The yield is 89.8%.
Apply[1]
2-Nitrophenyl silk cyanate can be used as a pharmaceutical synthesis intermediate. For example, prepare 1,2-bis(2-nitrophenyl)diselenide:
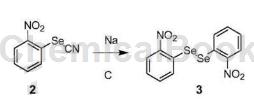
Metal sodium (6.0g, 261mmol) was added to a mixture of compound 2 (10.5g, 46.2mol) and absolute ethanol (520mL) at room temperature, and the resulting mixture was stirred in a water bath for 1 hour. Cool to 0-5°C, filter, wash the filter cake with a small amount of cold ethanol, then suspend the collected solid in toluene (100 mL), raise the temperature to reflux to dissolve the product, and then filter while hot. The filtrate is cooled to 0-5°C, solid is precipitated, filtered, and the filter cake is collected to obtain 1,2-bis(2-nitrophenyl)diselenide (3) (4.5g). The yield is 48.4%.
Main reference materials
[1] CN201610299350.1 Compounds for treating or preventing breast cancer

 微信扫一扫打赏
微信扫一扫打赏

