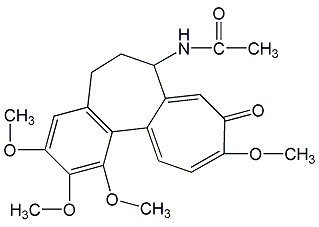
Structural formula
| Business number | 01DK |
|---|---|
| Molecular formula | C22H25NO6 |
| Molecular weight | 399.44 |
| label |
Colchicine, Treat breast cancer |
Numbering system
CAS number:64-86-8
MDL number:MFCD00078484
EINECS number:200-598-5
RTECS number:GH0700000
BRN number:2228813
PubChem number:24892656
Physical property data
1. Properties: Light yellow needle-like crystals or crystalline powder. 2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 155-157(dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): -250 º ( c=1, alcohol)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in Water (1g is soluble in 22ml water) and ether (1g is soluble in 220ml ether). Easily soluble in ethanol and chloroform.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 106.60
2. Molar volume (cm3/mol): 318.1
3. Isotonic specific volume (90.2K ): 848.1
4. Surface tension (dyne/cm): 50.5
5. Polarizability (10-24cm3): 42.25
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 2
6. Topological molecule polar surface area 83.1
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 740
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain atomic stereocentersQuantity: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent bonds Number of units: 1
Properties and stability
None yet
Storage method
Brown glass bottle with light-sealed packaging. Store in a cool and dry place.
Synthesis method
Colchicine can be extracted from Sagittarius sagittata as raw material.
Crush the Cigu into 40-80 mesh powder, feed 60-70kg per tank, add 180-210L of 85%-95% ethanol, heat and reflux for 4 hours, suction filter, and add ethanol reflux extraction, a total of 3 times, the third suction filtration mother liquor is applied, the first and second suction filtrate are concentrated under reduced pressure, and the ethanol is recovered. Add 3 to 5 times the amount of water to the distillation residue, stir evenly and let it stand. After the colloid precipitates, filter it with a cloth bag. Wash the colloid with water three times, and combine the washing liquid with the filtrate. Adjust the filtrate to pH=2 with 10% sulfuric acid, add chloroform and shake to extract until the chloroform extract is colorless. Combine the chloroform liquid and wash it once with about 300ml of 5% sodium hydroxide solution. Separate the chloroform layer and wash it with anhydrous sodium sulfate. Dehydrate and distill chloroform until nearly dry to obtain a gel. Add the obtained gel to 5 times the amount of ethyl acetate, heat to dissolve, then filter, leave to crystallize, filter with suction, and dry the crude product at a temperature below 60°C to obtain crude colchicine. Add 2 times chloroform to the crude colchicine, heat to dissolve and then filter. Recover 1/2 of the chloroform from the filtrate, leave it overnight, filter to dryness, evaporate the residual chloroform first, and then dry at low temperature to obtain colchicine salt crystals. Then add 5 times the amount of ethyl acetate, heat to dissolve, place to crystallize, filter, and dry the crystals below 60°C to obtain the finished product of colchicine.
Purpose
1. Mainly used to treat breast cancer, and also has certain effects on uterine cancer, esophageal cancer, lung cancer, and gastric cancer.

 微信扫一扫打赏
微信扫一扫打赏

