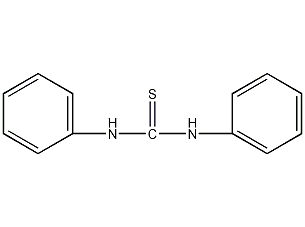
Structural formula
| Business number | 02LN |
|---|---|
| Molecular formula | C13H12N2S |
| Molecular weight | 228.31 |
| label |
N,N’-Diphenylthiourea, accelerator CA, diphenylamine thione, diphenylthiourea, diphenylthiourea, Diphenylthiourea, 1,3-Diphenyl-2-thiourea, Diphenylsulfourea, Thiocarbanilid, aromatic sulfur compounds |
Numbering system
CAS number:102-08-9
MDL number:MFCD00004921
EINECS number:203-004-2
RTECS number:FE1225000
BRN number:644277
PubChem number:24900084
Physical property data
1. Properties: off-white powdery crystal.
2. Relative density (g/mL, 20℃): 1.32
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (white flake crystal, ºC): 154-156
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa ): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º) : Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20.2ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): 80
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol and ether , completely insoluble in water and carbon disulfide. Soluble in dimethylformamide, acetone, chloroform, and benzene.
Toxicological data
1. Acute toxicity: LD5O: 50mg/kg (rat oral); LDLO: 720mg/kg (mouse oral); LDLO: 1500mg/kg (rabbit oral)
2 .Poisoning may cause insensitivity and peripheral nerve paralysis, but it does not cause distortion or cancer. This product produces phenyl mustard oil at temperatures above 160°C, which is highly irritating to eyes, skin, and mucous membranes.
Ecological data
General Notes
Do not allow this product to come into contact with groundwater, watercourses or sewage systems
Water Hazard Class 2 (German Regulation) (self-check via list)(Estimate) This substance is extremely harmful to water.
Even small amounts of product seeping into the ground can pose a hazard to drinking water.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 72.33
2. Molar volume (cm3/mol): 177.7
3. Isotonic specific volume (90.2K): 506.9
4. Surface tension (dyne/cm): 66.1
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 28.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 56.2
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 196
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1 Avoid contact with oxides. Easily soluble in ethanol and ether, completely insoluble in water and carbon disulfide. Soluble in dimethylformamide, acetone, chloroform, and benzene. Slightly soluble in various plasticizers used in polyvinyl chloride, dissolved in alkaline solutions and precipitated in acidic solutions. Bitter taste. Flammable. Glows when rubbed. Minimal toxicity.
2.Poisonous. Can cause serious injury or death if swallowed, inhaled or absorbed through skin contact. Contact of molten material with skin or eyes can cause severe burns. Suitable protective clothing should be worn.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should also be available to contain spills.
Synthesis method
Add 1.86 parts of aniline (98%), 1.0 parts of carbon disulfide (98%), and 1.0 parts of absolute ethanol (industrial product) into the reaction kettle, start stirring, and heat to reflux. Maintain the reflux condition for 12 hours. The reaction tail gas contains hydrogen sulfide and should be treated with alkali or lime water. The reaction product NNdiphenylthiourea is sent to the distillation device, heated to 100°C, and carbon disulfide and ethanol are steamed out under normal pressure (both are recycled). After the low boiling matter is evaporated, the residue is the crude N-N′-diphenylthiourea product. The crude product is discharged while hot and condenses into blocks after natural cooling. After crushing into powder, wash with dilute hydrochloric acid to remove alkaline substances, then wash with water until neutral, and then dry to get the finished product. 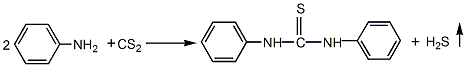
Purpose
Mainly used in natural rubber latex and neoprene dairy products, and also used in the manufacture of vulcanized capsules, water tires, tire repair rubber, wires and cables, industrial products, rubber shoes, etc. The general dosage is 3.5 to 4 parts, combined with 1.5 to 2 parts of sulfur. This product is also used as a heat stabilizer for polyvinyl chloride by emulsion polymerization. It is especially suitable for soft products. The general dosage is 0.2 to 0.5 parts. It will not color plastic products, but it cannot prevent the products from being discolored by light. It cannot be used together with lead, cadmium and other stabilizers, otherwise it will cause discoloration of the product. This product is an intermediate for medicines and dyes and a chemical reagent for the determination of osmium and rhodium products.

 微信扫一扫打赏
微信扫一扫打赏

