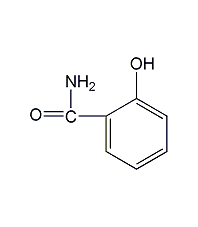
Structural formula
| Business number | 01DT |
|---|---|
| Molecular formula | C7H7NO2 |
| Molecular weight | 137.14 |
| label |
2-Hydroxybenzamide, Salicylamide, 2-Hydroxybenzamide, 2-(HO)C6H4CONH2 |
Numbering system
CAS number:65-45-2
MDL number:MFCD00007978
EINECS number:200-609-3
RTECS number:VN6475000
BRN number:742439
PubChem ID:None
Physical property data
1. Character:White or slightly pink crystalline powder. Slightly bitter
2. Density (g/mL,25/4℃):1.175
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):140-144 (lit.)
5. Boiling point (ºC,Normal pressure):270
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash Point (ºC,14mmHg):181
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
2. Molar volume (m3/mol):106.5
3. isotonic specific volume (90.2K):296.7
4. Surface Tension (dyne/cm):60.1
5. Polarizability(10-24cm3):14.69
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 7
6. Topological molecule polar surface area 63.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 136
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed, dry and protected from light.
Synthesis method
Combine methyl salicylate and 25% ammonia in 20℃ Stir closedly 3h, and then in30-40℃Stir5h. Cool and acidify the reaction solution to pH3-4, and salicylic acid crystals will precipitate. In industrial production, sodium metabisulfite can also be added to the aqueous solution of salicylic acid, and then passed through the ammonia reaction. The yield is 90% and above.
Purpose
for organic synthesis. Medical research. Anticorrosive.
This product is an antipyretic and analgesic drug, and is also used to prepare o-ethoxybenzamide (i.e., analgesic) and other drugs. Orally administered LD50 to mice is 1.4g/kg.
-kerning: 0pt”>
Synthesis method
Combine methyl salicylate and 25% ammonia in 20℃ Stir closedly 3h, and then in30-40℃Stir5h. Cool and acidify the reaction solution to pH3-4, and salicylic acid crystals will precipitate. In industrial production, sodium metabisulfite can also be added to the aqueous solution of salicylic acid, and then passed through the ammonia reaction. The yield is 90% and above.
Purpose
for organic synthesis. Medical research. Anticorrosive.
This product is an antipyretic and analgesic drug, and is also used to prepare o-ethoxybenzamide (i.e., analgesic) and other drugs. Orally administered LD50 to mice is 1.4g/kg.

 微信扫一扫打赏
微信扫一扫打赏

