Background and overview[1]
Methylbenzothiophene-2-carboxaldehyde is an organic synthesis intermediate that can be used in laboratory research and development processes and chemical and pharmaceutical synthesis. It can be obtained by esterification of benzothiophene-2-carboxylic acid.
![]()
Preparation[1]
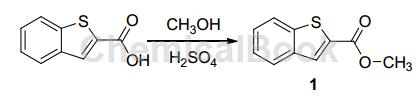
Dissolve 14.25 g (80 mmol) indole-2-carboxylic acid in 500 mL methanol, add 2 mL concentrated sulfuric acid, reflux for 24 hours at 75 °C, and monitor the reaction progress with TLC (5% water-acetonitrile). Reaction After completion, most of the solvent was evaporated under reduced pressure, and the solution was cooled and left to stand for about 10 hours at 0°C. Needle-like crystals precipitated and filtered to obtain 15.36 g of benzothiophene-2-carboxylic acid methyl ester (1), with a yield of 93%.
Apply[1]
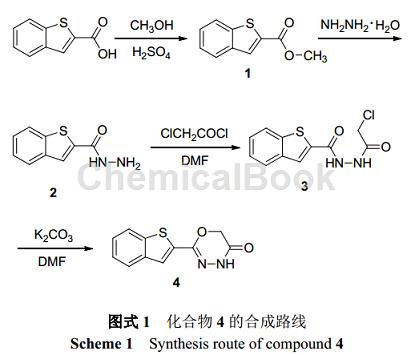
Can be used to synthesize 2-substituted-1,3,4-oxadiazinone compounds – compound 4. Such compounds have good herbicidal activity, especially against crabgrass (Digitaria sanguinalis) and amaranth (Ambrosia tricolor Linn).
1. Synthesis of compound 2
Dissolve 7.68 g (40 mmol) 1 in 500 mL methanol, add 23.12 g (0.36 mol) hydrazine hydrate, reflux the reaction at 80 °C for 20 h, and monitor the reaction with TLC (5% water-acetonitrile). After the reaction is completed , most of the solvent was evaporated under reduced pressure, and the solution was cooled and left to stand for about 10 hours at 0°C. Needle-like crystals precipitated and filtered to obtain 7.06 g of benzothiophene-2-carboxylic hydrazide (2), with a yield of 92%.
2. Synthesis of compound 3
Dissolve 4.5 g (25 mmol) 2 in 160 mL DMF, add 2.92 g (27.5 mmol) triethylamine, cool the solution to 0 ℃, slowly add 4.90 g (37.5 mmol) chloroacetyl chloride, 0 ℃ React for 4 hours, and monitor the reaction with TLC (50% ethyl acetate-petroleum ether). After the reaction, filter to remove the white precipitate, evaporate about 2/3 of the solvent under reduced pressure, cool and let stand at room temperature if solid precipitates, filter A white solid was obtained, which was recrystallized from ethyl acetate to obtain 5.36 g of 1-(benzothiophene-2-formyl)-2-(chloroacetyl)hydrazine (3), with a yield of 80%.
3. Synthesis of compound 4
Heat and dissolve 2.69 g (10 mmol) 3 in 50 mL DMF, add 6.9 g (50 mmol) potassium carbonate, react at 80 °C for 6 h, and monitor the reaction with TLC (50% ethyl acetate-petroleum ether). After the reaction is completed, filter to remove the precipitate. Add 50 mL of water to the filtrate. The solid will immediately precipitate into a suspension. Cool and let stand overnight. Filter to obtain a light yellow solid 2-(2-benzothienyl)-4H-1. ,3,4-oxadiazin-5(6H)-one(4) 1.11 g, yield 48%.
Main reference materials
[1] Sun Hongshun, Wang Jianqiang, Tan Wenwen, Yang Haidong, Li Yulong, Shen Linjiang, Guo Cheng. Synthesis and crystal structure of a new 1,3,4-oxadiazinone compound [J]. Organic Chemistry, 2016, 36(03):622-625.

 微信扫一扫打赏
微信扫一扫打赏

