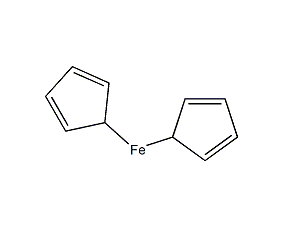
Structural formula
| Business number | 02M8 |
|---|---|
| Molecular formula | C10H10Fe |
| Molecular weight | 186.03 |
| label |
bis(cyclopentadienyl)iron, Di(cyclopentadienyl)iron, Bis(cyclopentadienyl)iron, Di(cyclopentadienyl)iron, catalyst, reducing agent |
Numbering system
CAS number:102-54-5
MDL number:MFCD00001427
EINECS number:203-039-3
RTECS number:LK0700000
BRN number:None
PubChem number:24869986
Physical property data
1. Properties: It is an orange-yellow powder at room temperature and has a camphor smell.
2. Density (g/mL, 25℃): 0.997
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 172-174
5. Boiling point (ºC, normal pressure): 249
6. Boiling point (ºC, mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 40ºC): 0.03
12. Saturated vapor pressure (kPa, ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, easily soluble in organic solvents such as benzene and ether.
Toxicological data
1. Acute toxicity: Rat oral LD5O: 1320mg/kg; Mouse oral LD5O: 832mg/kg
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 11.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters.�0
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The uncertainty of chemical bond stereocenters Number of structural centers: 0
15, number of covalent bond units: 3
Properties and stability
1. Avoid contact with oxidants.
2. The chemical properties of this reagent are stable and will not decompose within 400 oC. When heated above 400 oC, it will decompose and release spicy and irritating substances. of smoke. It is not sensitive to air and moisture, and does not react easily with acid or alkali, but it is sensitive to oxidants. In addition, inhalation or skin contact should be avoided as prolonged exposure can cause liver damage.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. Protect from direct sunlight. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is obtained by heating iron powder and cyclopentadiene in a nitrogen atmosphere at 300°C, or by reacting anhydrous ferrous chloride and sodium cyclopentadiene in tetrahydrofuran. It can also be synthesized by electrolysis. , using cyclopentadiene, ferrous chloride, and diethylamine as raw materials to synthesize ferrocene can be performed as follows. While stirring, add anhydrous ferric chloride (FeCL3) into tetrahydrofuran in portions, then add iron powder, and heat and reflux under nitrogen protection for 4.5 hours to obtain a ferrous chloride solution. The solvent tetrahydrofuran was evaporated under reduced pressure to obtain a nearly dry residue. While cooling in an ice bath, add a mixture of cyclopentadiene and diethylamine, stir vigorously at room temperature for 6-8 hours, evaporate excess amine under reduced pressure, and extract the residue with petroleum ether reflux. Filter the extract while it is hot and evaporate the solvent to obtain crude ferrocene. Use pentane or cyclohexane to recrystallize, or use sublimation method to extract the pure product, which is a refined product yield of 73-84%.
2. In a 250 ml three-neck flask equipped with a mechanical stirrer, reflux condenser and nitrogen introduction tube, add 100 ml of pure Tetrahydrofuran, add 27.1 grams (0.166 mol) anhydrous ferric chloride and 4.7 grams (0.084 grams atoms) iron powder in portions while stirring. The reaction was heated and refluxed for 4.5 hours under stirring and nitrogen flow, and a gray powder with a brown clear liquid as the upper layer was obtained.
At the same time, a reflux condenser equipped with a mechanical stirrer and a calcium chloride drying tube connected to the xylene bubble meter is installed on the top. And into a 500 ml three-neck flask with a pressure dropping funnel that can be passed into nitrogen flow, add 200 ml of sodium dry xylene and 11.5 grams (0.5 grams atom) of metallic sodium. Bring to a boil, stirring quickly to disperse the sodium well. Continue stirring and purging nitrogen, allowing the mixture to cool and settle, and most of the xylene is sucked out of the cylinder. Add 200 ml of tetrahydropyran through the funnel, and add 42 ml (0.5 mol) of freshly steamed cyclopentadiene in portions under stirring and ice cooling for about 1 hour. Continue the reaction with stirring and cooling for 2 to 3 hours.
Under nitrogen, add the contents of the above 250 ml flask containing ferrous chloride to the cooled sodium cyclopentadienyl solution, and stir the reaction just below the reflux temperature for 1.25 hours. The solvent is evaporated, and several portions of hot petroleum ether are used to extract ferrocene from the residue. The petroleum ether is evaporated to obtain the product. Ferrocene can be purified by recrystallization from pentane or cyclohexane, or by sublimation. Get 31-34 grams (67-73%).
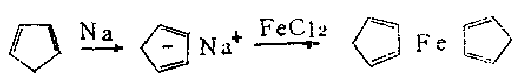
3. In a 250 ml flask, prepare 0.25 mol of ferrous chloride in 100 ml of tetrahydropyran as above suspension in. The tetrahydropyran was evaporated under reduced pressure until it was almost dry. Place the flask in an ice bath to cool, add a mixture of 42 ml (0.5 mol) of freshly steamed cyclopentadiene and about 100 ml (approximately 1 mol) of diethylamine, and stir vigorously at room temperature for 6 to 8 hours. Excess amine was removed under reduced pressure, and the residue was repeatedly extracted with petroleum ether under reflux. After hot filtration of the extract, the solvent is evaporated to obtain ferrocene orange crystals, which can be recrystallized in pentane or cyclohexane or further purified by sublimation. The product weighs 34 to 39 grams (73 to 84%)
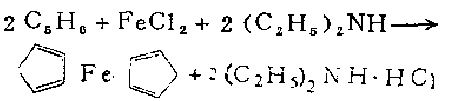
Purpose
Ferrocene has very extensive and important uses in the fields of chemistry, industry and medicine, such as: it can be used as a fuel smoke suppressant or Burning rate regulator, photosensitizer, stabilizer, polymer material improver, etc., and can be used to synthesize drug intermediates such as D-alanine, ferrocene penicillin, etc., and can also be used as a blood supplement to treat iron deficiency anemia, etc. .
Ferocene is a special metal-organic compound. Its solubility in organic solvents, its electron-rich characteristics and “sandwich-type” structure make it an important component in organic synthesis. It has two uses: (1) directly used as reducing agent or catalyst; (2) preparation of ferrocene derivatives through functionalization of cyclopentadiene ring.
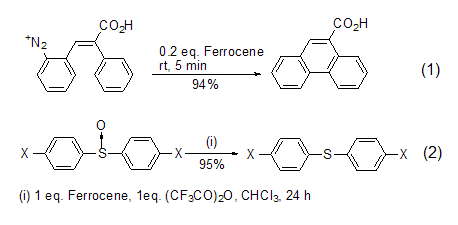
Ferocene itself is a good reducing agent and can participate in many redox reactions. For example, it can be used as a single-electron homogeneous catalyst to catalyze the Pschorr cyclization reaction, which would otherwise require The reaction of several hours is completed within a few minutes, and a higher yield is obtained (Formula 1)[1]. It can also reduce sulfoxide to sulfide in the presence of trifluoroacetic anhydride. (Formula 2)[2].
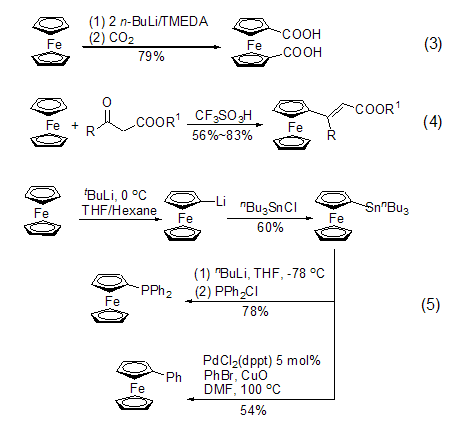
By activating the cyclopentadiene ring of ferrocene, a series of derivatives can be prepared (Formula 3[3], Formula 4[4], Formula 5 [5] ), some of the derivatives with planar chirality can be used as chiral ligands to coordinate with other metals to form chiral catalysts. Already realized reactions such as the asymmetric Diels-Alder reaction[6,7], asymmetric allylation reaction[8,9], and some other asymmetric functionalization reactions[10] .
In addition, ferrocene is It has also been widely used in molecular chemistry and nanoscience[11,12].
It is completed within a few minutes and obtains a higher yield (Formula 1)[1]. It can also reduce sulfoxide to sulfide in the presence of trifluoroacetic anhydride (Formula 2) [2].

By activating the cyclopentadiene ring of ferrocene, a series of derivatives can be prepared (Formula 3[3], Formula 4[4], Formula 5 [5] ), some of the derivatives with planar chirality can be used as chiral ligands to coordinate with other metals to form chiral catalysts. Already realized reactions such as the asymmetric Diels-Alder reaction[6,7], asymmetric allylation reaction[8,9], and some other asymmetric functionalization reactions[10] .

In addition, ferrocene is It has also been widely used in molecular chemistry and nanoscience[11,12].

 微信扫一扫打赏
微信扫一扫打赏

