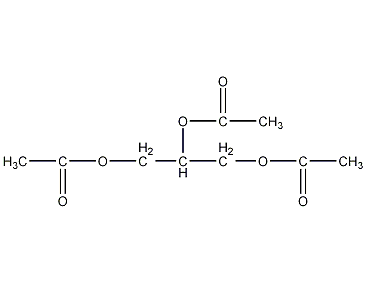
Structural formula
| Business number | 02ME |
|---|---|
| Molecular formula | C9H14O6 |
| Molecular weight | 218.20 |
| label |
1,2,3-propanetriol triacetate, triacetin, 1,2,3-Propanetriol triacetate, Triacetyl glycerine, plasticizer, spice fixative, Raw materials and intermediates used in ink |
Numbering system
CAS number:102-76-1
MDL number:MFCD00008716
EINECS number:203-051-9
RTECS number:AK3675000
BRN number:1792353
PubChem ID:None
Physical property data
1. Properties: colorless oily liquid with a faint fruity aroma and sweet meaty taste, and a bitter taste at low concentrations.
2. Density (g/mL, 20/4℃): 1.1583
3. Relative vapor density (g/mL, air=1): 7.52
4 . Melting point (ºC): -78
5. Boiling point (ºC, normal pressure): 258~260
6. Refractive index (n20ºC): 1.4301
7. Flash Point (ºC, closed): 138
8. Flash point (ºC, open): 148
9. Viscosity (mPa·s, 25ºC): 16.1
10. Flash point (ºC): 433
11. Vapor pressure (kPa, 100ºC): 0.133
12. Vapor pressure (kPa, 60ºC): 0.00666
13. Heat of evaporation (KJ/mol, 25ºC): 82.1
14. Solubility: Soluble in alcohol, ether, benzene, chloroform and castor oil, but insoluble in linseed oil. Can dissolve nitrocellulose, cellulose acetate, acrylic resin, polyvinyl acetate, etc. It can also dissolve natural rosin to a certain extent, but is not miscible with polyvinyl chloride, polystyrene, and chlorinated rubber.
15. Relative density (20℃, 4℃): 1.1596
16. Refractive index at room temperature (n25): 1.4283
17. Solubility parameter (J·cm-3)0.5: 20.136
18. van der Waals area (cm2 ·mol-1): 1.620×1010
19. van der Waals volume (cm3·mol-1): 114.000
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4293.6
21. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -1248.8
22. Liquid phase standard combustion heat (enthalpy) (kJ ·mol-1): -4211.6
23. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -1330.8
24. Liquid phase standard hot melt (J·mol-1·K-1): 379.2
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit eye contact, 116mg; 2. AcuteProperties: Rat oral LD50: 3mg/kg; Rat peritoneal cavity LD50: 2100mg/kg; Rat subcutaneous LD50: 2800mg/kg; Mouse oral LD50: 1100mg/kg; Mouse peritoneal cavity LD50: 1 400mg /kg; Mouse subcutaneous LD50: 2300mg/kg; Mouse intravenous LD50: 1600mg/kg; Dog intravenous LD50: 1500mg/kg; Rabbit intravenous LD50: 750mg/kg; Guinea pig muscle LDLo: 1704mg/kg; Amphibians – Oral LDLo: 150mg/kg;
Ecological data
This substance may be harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 49.02
2. Molar volume (cm3/mol): 187.9
3. Isotonic specific volume (90.2K): 461.7
4. Surface tension (dyne/cm): 36.4
5. Polarizability: 19.43
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 78.9
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 229
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants. 2. Miscible with organic solvents such as methanol and ethanol, soluble in acetone, and insoluble in mineral oil. Slightly soluble in water, solubility in water at 25°C is 5.9g/100ml. Non-toxic. 3. Exist in smoke.
Storage method
Packed in galvanized iron drum. Store in a cool and dry place, pay attention to sun and fire protection.
Synthesis method
1. Obtained from the esterification of glycerin and acetic acid. After preheating the glycerol to 50-60°C, add acetic acid, benzene and sulfuric acid. Heat, stir, reflux and dehydrate. After dehydration, recover benzene, then add acetic anhydride and heat for 4 hours. After cooling, neutralize the pH to 7 with 5% sodium carbonate, separate the water layer, and dry the crude oil with calcium chloride. Distill under reduced pressure and collect the 128-131°C (0.93kPa) fraction, which is glycerol triacetate. 2. Obtained from the esterification of acetic anhydride and hot glycerin, followed by vacuum distillation and purification. 3.Esterification reaction: Add a certain amount of glycerol, glacial acetic acid (alkyd-to-acid molar ratio: 1:4), and solid-supported heteropolyacid catalyst ( 2.5% of the alcohol mass) and the water-carrying agent toluene (temperature regulator), heat the tower still liquid, so that the still liquid is in a slightly boiling or boiling state, and carry out reactive distillation operation. The ester phase in the water separator at the top of the tower returns to the distillation tower, and the water phase is extracted. When no water droplets separate from the water separator at the top of the tower, add a certain amount of acetic anhydride to the tower kettle. After continuing the reaction for 4 hours, the esterification reaction is terminated. After cooling, the crude ester is obtained. 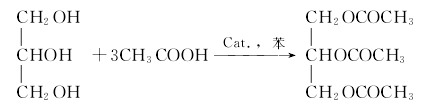 4.Crude ester The refined crude ester is cooled and the solid catalyst filtrate is filtered out. First perform atmospheric distillation to recover excess glacial acetic acid and water-carrying agent toluene. When the distillate is very small, use vacuum distillation to further remove glacial acetic acid. Control the temperature of the still liquid within 100 to 120°C. Stop the distillation operation when the distillate is distilled; the temperature of the kettle liquid drops to within 100°C, add a certain amount of decolorizing agent activated carbon, stir for a certain period of time, cool, filter out the decolorizing agent, and obtain the product triacetin.
4.Crude ester The refined crude ester is cooled and the solid catalyst filtrate is filtered out. First perform atmospheric distillation to recover excess glacial acetic acid and water-carrying agent toluene. When the distillate is very small, use vacuum distillation to further remove glacial acetic acid. Control the temperature of the still liquid within 100 to 120°C. Stop the distillation operation when the distillate is distilled; the temperature of the kettle liquid drops to within 100°C, add a certain amount of decolorizing agent activated carbon, stir for a certain period of time, cool, filter out the decolorizing agent, and obtain the product triacetin.
Purpose
1. Can be used as a plasticizer for cellulose resins and vinyl copolymers. It can also be used to plasticize cellulose acetate to make cigarette filters, providing good elasticity, breathability and appropriate hardness. It can be used as a solvent and carrier in the pharmaceutical industry. It can be used as solvent and fixative in perfumery. It can also be used in industrial products such as plastics. Used as a solvent for celluloid, photographic films, preservatives, etc. Also used as plasticizer and spice fixative.
2.Plasticizers for adhesives, cigarette filters, and some plastic products. It can also be used as a food additive, chemical solvent, spice fixative, swelling agent and stabilizer for cellulose acetate in the printing and dyeing industry, determination of lipase substrate and gas chromatography fixative, etc.
3. Can be used in daily chemical flavor formulas and food flavor formulas.

 微信扫一扫打赏
微信扫一扫打赏

