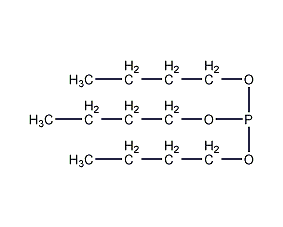
Structural formula
| Business number | 02MM |
|---|---|
| Molecular formula | C12H27O3P |
| Molecular weight | 250.31 |
| label |
Tributyl phosphite, Phosphorous Acid Tributyl Ester |
Numbering system
CAS number:102-85-2
MDL number:MFCD00009437
EINECS number:203-061-3
RTECS number:TH0875000
BRN number:1703866
PubChem number:24862021
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25℃): 0.925
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -80
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 0.95kPa): 118-125
7. Refractive index: 1.431
8. Flash point (ºC): 121
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in most organic solvents.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 10mg/24H; Standard Dresser test: rabbit skin contact, 5mg/24HREACTION SEVERITY, strong reaction; Standard Dresser test: rabbit eye contact, 500mgREACTION SEVERITY , moderate reaction; 2. Acute toxicity: Rat oral LD50: 3mg/kg; Rat inhalation LC: >220ppm/6H; Rabbit skin contact LD50: 2mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 12
5.��Number of isomers: None
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 110
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong alkali, and water.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, alkalis, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
In a reaction bottle equipped with a stirrer, reflux condenser and thermometer, add 74g (1.0mol) of 1-butanol (2) and 79g (1.0mol) of pyridine ), 250 mL of dry ether, cooled in an ice-salt bath, kept at about 0°C, and a mixture of 46 g (0.33 mol) of phosphorus trichloride and 30 mL of ether was added dropwise. After the addition was completed, the reaction was continued to stir for 0.5 h. Pyridine hydrochloride was filtered off, and the filter cake was washed with diethyl ether. Combine the filtrate and washing liquid and dry over anhydrous sodium sulfate. First steam off the ether, then distill under reduced pressure, collect the 105~110℃/67Pa (or 122℃/1.6kPa) fraction, and obtain 75g of tributyl phosphite (1), with a yield of 90%. [1]
2. Preparation method:
Equipped with stirrer, thermometer, dropping funnel, reflux condensation In the reaction bottle of the reactor, add 138g (3.0mol) of absolute ethanol (2) and 100mL of benzene, and cool to 10°C in an ice-water bath while stirring. Slowly add a mixture of 137g (1.0mol) of newly distilled phosphorus trichloride and 100mL benzene dropwise, keeping the reaction temperature between 5 and 10°C, and complete the addition in about 1 hour. After the addition was completed, the reaction was stirred at room temperature for 3 hours. Heating in a 40°C water bath, removing hydrogen chloride under reduced pressure, and then distilling under reduced pressure, collecting the fraction at 74~75°C/1.87kPa to obtain 124g of diethyl phosphite ① (2), yield 90%. Note: ① Referring to the above method, various dialkyl phosphites can be prepared as follows: dimethyl phosphite (C2H7O3P): 56~58℃/1.33kPa, yield 60%~80%; dibutyl phosphite ( C8H19O3P): 124~125℃/1.6kPa, yield 80%~90%; diisobutyl phosphite (C8H19O3P): 106~107℃/1.6kPa, yield 80%~90%; dipropyl phosphite (C6H15O3P): 91~92℃/1.45kPa; diisopropyl phosphite (C6H15O3P): 83~84℃/2.26kPa. [2]
Purpose
Used as enhancer, preservative and heat stabilizer for lubricating oil

 微信扫一扫打赏
微信扫一扫打赏

