Background and overview[1]
Phenylthiohydantoin-tryptophan is used as a pharmaceutical synthesis intermediate. If phenylthiohydantoin-tryptophan is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
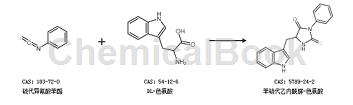
Synthesis of phenylthiohydantoin-tryptophan via phenylthioisocyanate and DL-tryptophan:
Related research
Ion mobility mass spectrometry (IMMS) can rapidly (<1 minute) differentiate 20 phenylisothiocarbamoyl/phenylthioisocyanate amino acid mixtures including phenylthio Hydantoin-Tryptophan while still maintaining high detection (<17.0 pmol):
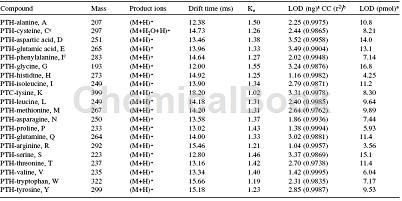
All 20 phenylisothiocarbamoyl/phenylthioisocyanate amino acids including phenylthiohydantoin-tryptophan can be isolated in less than a minute, although this has not been studied with reagents There are issues associated with ion suppression, but the results of the initial evaluation of IMMS for the rapid separation and identification of phenylisothiocarbamoyl/phenylthioisocyanato amino acids are promising. IMMS technology, operating as a stand-alone instrument or combined with a high-speed liquid chromatography system, provides additional separation power and speed for the determination of complex mixtures such as phenylisothiocarbamoyl/phenylthioisocyanato-amino acids. When applied to phenylisothiocarbamoyl/phenylthioisocyanato amino acids separation and detection, IMMS will increase sample throughput by eliminating or reducing the rate-limiting step of chromatography in protein microsequencing.
Main reference materials
[1] Rapid separation of phenylthiohydantoin amino acids: ambient pressure ion-mobility mass spectrometry (IMMS)

 微信扫一扫打赏
微信扫一扫打赏

