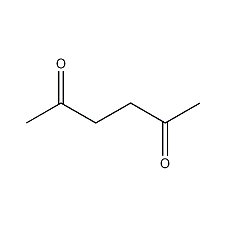
Structural formula
| Business number | 030L |
|---|---|
| Molecular formula | C6H10O2 |
| Molecular weight | 114 |
| label |
Acetone acetone, diacetone, hexanedione, 2,5-Diketohexane, 2,5-Hexadione, α,β-Diacetylethane, 1,2-Diacetylethane |
Numbering system
CAS number:110-13-4
MDL number:MFCD00008792
EINECS number:203-738-3
RTECS number:MO3150000
BRN number:506525
PubChem number:24849833
Physical property data
1. Properties: colorless flammable liquid with slight odor, gradually turning yellow in the air.
2. Relative density (g/mL, 20/20℃): 0.737
3. Relative vapor density (g/mL, air=1): 3.94
4. Melting point (ºC): -6~-4
5. Boiling point (ºC, normal pressure): 194
6. Boiling point (ºC, kPa): Not available Confirm
7. Refractive index(n20D): 1.4232
8. Flash Point (ºC): 80
9. Specific rotation (º): Undetermined
10. Flash point (ºC): 504
11. Vapor pressure (mmHg, 20ºC): 0.43
12. Saturated vapor pressure (kPa, 20ºC): 0.057
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient : Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: miscible with water and ethanol and ether, but not miscible with hydrocarbon solvents.
Toxicological data
It is not harmful to human body. Its vapor is irritating to mucous membranes, and long-term skin contact with it can cause dermatitis due to fat solubility. The oral LD50 in rats is 2.7g/kg, and the transdermal LD50 in guinea pigs is 6.426mg/kg. The maximum allowable concentration in the workplace is 349.5mg/m3.
Ecological data
This substance is harmful to the environment and special attention should be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 29.90
2. Molar volume (cm3/mol): 121.8
3. Isotonic specific volume (90.2K ): 280.9
4. Surface tension (dyne/cm): 28.3
5. Polarizability (10-24cm3): 11.85
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 6
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 91.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Prohibit contact with strong oxidants, strong reducing agents, and strong alkali. It has a high boiling point and low vapor pressure. It is relatively safe to use under normal conditions, but you still need to pay attention to fire prevention. Miscible with water, ethanol and ether, but not miscible with hydrocarbon solvents. It is also not soluble in concentrated potassium hydroxide or potassium carbonate solutions. It can dissolve synthetic resins such as polymethyl methacrylate, polystyrene, polyvinyl acetate, vinyl chloride and vinyl acetate copolymer, nitrocellulose, phenolic resin, and natural resins such as rosin, kauri, and resin. Shellac, dewaxed dammar resin, glyceryl trirosinate, cottonseed oil, etc. can be partially dissolved.
2. Chemical properties: It has the general reaction of ketones. Reacts with phosphorus pentasulfide to form 2,5-dimethylthiophene. When reacting with ammonia alcohol solution or heating with ammonium carbonate, 2,5-dimethylpyrrole is generated. Dehydration produces 2,5-dimethylfuran. It reacts with 2,4-dinitrophenylhydrazine to form bis(2,4-dinitrophenyl)hydrazone with a melting point of 257°C.
3. Exist in flue-cured tobacco leaves and smoke.
4. A moderate local irritant that can cause anesthesia at high concentrations.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Storage temperature 1.449ºC
Synthesis method
1. Obtained from the hydrolysis of 2,5-dimethylfuran. Another synthesis method is to react sodium ethyl acetoacetate with pure iodine to form diethyl diacetyl succinate, which is then hydrolyzed with 10% sodium hydroxide solution. The reaction solution was saturated with anhydrous potassium carbonate to precipitate acetone. Extract with diethyl ether, evaporate the diethyl ether from the extract, distill the residue, and collect the 192-194°C fraction to obtain a colorless product.
2. Refining method: The main impurities are water and acidic impurities. The refining method is drying with anhydrous calcium sulfate or anhydrous sodium sulfate and then distilling.
3. Tobacco: FC, 40.
4. Preparation method:
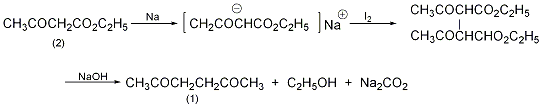
Diethyl diacetyl succinate (3): In a reaction bottle equipped with a stirrer, dropping funnel, and reflux condenser (with a calcium chloride drying tube on the top), add 300 mL of anhydrous ether, metal 4.5g (0.2mol) sodium wire, slowly add 26g (0.2mol) acetoethyl ester (2) dropwise while cooling, control the dropping speed, do not make the reaction too violent, and do not boil the ether. After adding, leave it overnight to form a white gel-like precipitate. Slowly add a solution of 25g (0.1mol) of finely ground iodine in 75mL of anhydrous ether while stirring until the solution completely fades (with a trace of iodine color). Filter to remove sodium iodide. The diethyl ether was evaporated, and the residue was recrystallized with glacial acetic acid to obtain 16.5g of colorless crystalline diethyl diacetyl succinate (3), mp78°C, yield 65%. Add 16.5g of compound (3) into the reaction bottle, then add 160mL of 10% sodium hydroxide solution, and reflux for 3 to 4 hours. After cooling, the alkaline solution was saturated with anhydrous potassium carbonate to precipitate an oily substance. Extract three times with diethyl ether. After distilling off the diethyl ether, purify by distillation and collect the fraction at 192-195°C to obtain 4.8g of colorless liquid acetonyl acetone (1) with a yield of 65%. [1]
5. Preparation method:
Put 3-hexyne-2,5-diol (2 ) 228mg (2mmol), IrH5 (i-Pr3P) 2 41mg (0.04mmol), and 10mL toluene were placed in a sealed tube and reacted at 110°C for 40h. Cool to room temperature and evaporate the solvent under reduced pressure. Distill the remaining red substance under reduced pressure and collect the fraction at 90°C/266Pa to obtain colorless 2,5-hexanedione with a yield of 70%. [2]
Purpose
It is used as a high-boiling point solvent for synthetic resins, nitrocellulose spray paints, colorants, printing inks, etc., leather tanning preparations, rubber vulcanization accelerators, and raw materials for manufacturing pesticides, pharmaceuticals, etc.

 微信扫一扫打赏
微信扫一扫打赏

