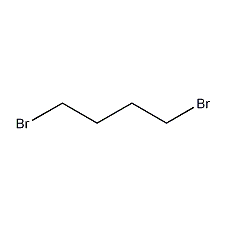
Structural formula
| Business number | 031C |
|---|---|
| Molecular formula | C4H8Br2 |
| Molecular weight | 215.93 |
| label |
Butylene dibromide, Tetramethyl bromide, propylene bromide, Tetramethylenyl dibromide, Tetramethylene dibromide, tetramethylene bromide, Butylene dibromide, 1,4-Butylene bromide, Tetramethylene dibromide, linear compound |
Numbering system
CAS number:110-52-1
MDL number:MFCD00000261
EINECS number:203-775-5
RTECS number:EJ7565000
BRN number:1071199
PubChem number:24848480
Physical property data
1. Properties: colorless or light yellow liquid.
2. Density (g/mL, 25℃): 1.808
3. Relative density (20℃, 4℃): 1.8265
4. Melting point (ºC): -20
5. Boiling point (ºC, normal pressure): 197.5
6. Relative density (25℃, 4℃): 1.8187
7. Refractive index at room temperature (n20): 1.5190
8. Flash point (ºC): >110
9. Refractive index at room temperature (n 25): 1.5169
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -87.0
11. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -140.1
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined Determined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in chloroform, alcohol and ether, insoluble in water.
Toxicological data
None yet
Ecological data
This substance is not harmful to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 36.04
2. Molar volume (cm3/mol): 120.9
3. Isotonic specific volume (90.2K): 295.5
4. Surface tension (dyne/cm): 35.6
5. Polarizability (10-24cm3): 14.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 17.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable in nature, it will not decompose under normal temperature and pressure. Avoid contact with oxides.
2. It is locally irritating and has an anesthetic effect at high concentrations.
Storage method
1. Store in a cool, ventilated warehouse, away from fire. Keep container tightly sealed and store away from oxidizing agents. Emergency release equipment and suitable containment materials should be available.
2. Packed in 250kg iron drum.
Synthesis method
1, obtained from tetrahydrofuran through ring opening and bromination. Add hydrobromic acid to the reaction pot, add tetrahydrofuran dropwise while stirring, and then add sulfuric acid dropwise. The temperature is controlled at 50℃below. Then heat up to108℃Reflux around4h, continue stirring after the reaction is completed 1h, let cool to separate the supernatant, wash with soda ash solution until neutral, add anhydrous calcium chloride to dry, filter to obtain the finished product.
![]()
2, by1,4- Butanediol is obtained by bromination. Add hydrobromic acid into the reaction pot, stir and cool until 10℃, add sulfuric acid dropwise. After the addition is completed, add 1,4- butanediol dropwise, and the temperature should not exceed 15℃, stir for half an hour. in100-110℃ insulation 6h. Cool to room temperature, separate the supernatant, and wash with soda ash solution until neutral. After dehydration of calcium chloride, 1,4-dibromobutane is obtained by filtration.
![]()
3. Preparation method: span>
![]()
In a reaction bottle equipped with a stirrer, reflux condenser, and dropping funnel, add 154g (105mL) of 48% hydrobromic acid. Cool in an ice-water bath, and slowly add 70 mL of concentrated sulfuric acid while stirring. Add 30g (0.33mol) of newly distilled 1,4-butanediol (2) dropwise, and leave it at room temperature for 24 hours after the addition. The reaction was heated in a boiling water bath for 3 hours. The reaction mixture was divided into two layers, and the lower layer was separated, washed with water, 5% sodium carbonate, and water in sequence, and dried over anhydrous magnesium sulfate. Distill under reduced pressure and collect the distillate of Kuli at 83~84℃/1.6kPa to obtain 55g of 1,4-dibromobutane (1) with a yield of 76%. [1]
Purpose
Organic synthesis intermediates, used in the manufacture of aminophylline, Kebiqing, Ququjing, etc. Used in the manufacture of aminophylline, Kebiqing, Qiqujing, etc.

 微信扫一扫打赏
微信扫一扫打赏

