Background and overview[1]
2,6-di-tert-butyl-4-mercaptophenol can be used as a pharmaceutical synthesis intermediate and can be used in laboratory research and development and chemical and pharmaceutical synthesis processes.
Preparation[1]
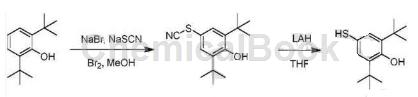
Step 1: 2,6-di-tert-butyl-4-thiocyanatophenol
Add 2,6-di-tert-butylphenol (2.06g, 10mmol), NaBr (1.02g, 10mmol), and NaSCN (1.62g, 20mmol) to a three-necked flask containing MeOH (30mL), respectively. The mixture was cooled in an ice-water bath, keeping the temperature at 0 to 5°C. Then a solution of Br2 (0.56 mL, 11 mmol) in MeOH (5 mL) was slowly added dropwise, and the temperature was controlled not to exceed 5°C. After the dropwise addition was completed, the resulting mixture was naturally raised to room temperature under stirring and monitored by TLC. Concentrate under reduced pressure to remove methanol, add H2O (100mL) to the residue, extract with EtOAc (100mL×3), and dry over Na2SO4. Concentrate under reduced pressure to remove EtOAc, and the crude product 2,6-di-tert-butyl-4-thiocyanatophenol is directly used in the next step.
Step 2: 2,6-di-tert-butyl-4-mercaptophenol
At 0°C, a solution of 2,6-di-tert-butyl-4-thiocyanatophenol (10.0g, crude product) in dry THF (30mL) was slowly added dropwise to lithium aluminum tetrahydrogen (2.0g , 52.6 mmol) in a suspension in THF (50 mL). Keep the reaction at 0°C for 5 hours, slowly add EtOAc (20 mL) to quench the reaction, then add 3NHCl (50 mL) and EtOAc (200 mL), and separate the organic phase. The organic phase was washed with saturated NaHCO3 and brine respectively, and dried over anhydrous sodium sulfate. The crude product was passed through a chromatography column (silica gel, EtOAc:PE=1:10 to 1:5) to obtain the product 2,6-di-tert-butyl-4-mercaptophenol (5.4g), a yellow waxy solid.
Apply[1]
2,6-di-tert-butyl-4-mercaptophenol can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
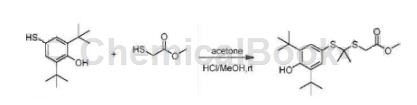
Methyl 2-mercaptoacetate (1 mL) and 2,6-di-tert-butyl-4-mercaptophenol (0.88g, 3.7mmol) were added to dry acetone (20 mL) at 0°C. Then slowly add MeOH (20 mL), and then slowly add HCl gas until the pH reaches 2~3. The reaction solution was allowed to react overnight at room temperature. The organic solvent was removed under reduced pressure. EtOAc (100 mL) was added to the residue, washed with saturated NaHCO3 and brine, and dried over anhydrous sodium sulfate. The crude product is passed through a chromatography column (silica gel, EtOAc: PE=1:20 to 1:5) to obtain the product methyl 2-((2-((3,5-di-tert-butyl-4-hydroxyphenyl) Thia)propyl-2-yl)thia)acetate (444 mg, yield 22%), colorless liquid, solidifies slowly at room temperature.
1HNMR (300MHz, CDCl3): δ7.30(s, 2H), 5.37(s, 1H), 3.74(s, 3H), 3.58(s, 3H), 3.88(s, 2H), 1.54(s , 6H), 1.44(s, 18H).
Main reference materials
[1] CN201810090320.9 A kind of probucol derivative and its preparation method and application

 微信扫一扫打赏
微信扫一扫打赏

