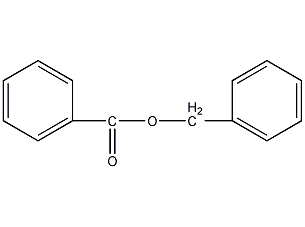
Structural formula
| Business number | 03CB |
|---|---|
| Molecular formula | C14H12O2 |
| Molecular weight | 212.24 |
| label |
Benzyl Benzoate, Benzyl benzoate, 3-Piperidine phenylpropionone hydrochloride, Benzyl benzoate, Benzoic acid phenylmethyl ester, musk solvent, fragrance fixative, plasticizer, insect repellent, Acaricide |
Numbering system
CAS number:120-51-4
MDL number:MFCD00003075
EINECS number:204-402-9
RTECS number:DG4200000
BRN number:2049280
PubChem number:24891923
Physical property data
1. Properties: Leaf-like crystals or colorless oily liquid. Has a faint balsamic aroma. There is a burning smell. Can evaporate with water vapor.
2. Relative density (g/mL, 18/4℃): 1.1121
3. Relative density (20℃, 4℃): 1.112125
4. Melting point (ºC): 21
5. Boiling point (ºC, normal pressure): 323.5~324
6. Relative density (25℃ , 4 ℃): 1.1085
9. Viscosity (mPa·s, 20ºC): 8.45
10. Refractive index at room temperature (n20): 1.5680
11. Room temperature Refractive index (n25): 1.5672
12. Liquid phase standard hot melt (J·mol-1·K-1): 332.8
13. Heat of evaporation (KJ/mol, 12~60ºC): 77.9
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Conductivity (S/m, 25ºC): <1×10-9
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Can be compared with Ethanol, chloroform, ether and oils are miscible, but insoluble in water and glycerol.
Toxicological data
1. Acute toxicity: rat oral LD50: 1700 μL/kg
Rat subcutaneous LD50: 4mL/kg
Mouse oral LC50: 1400 μL/kg
Mouse abdominal LC50: >500mg/kg
Dog oral LD50: >33440mg/kg
Cat oral LD50: 2240mg/kg
Rabbit oral LD50: 1680mg/kg
Rabbit subcutaneous LD50: 4g/kg
Guinea pig oral LD50: 1mL/kg
2. Other multi-dose toxicity: rabbit subcutaneous TDLO: 180mL/kg/13W-I;
3. General use is not dangerous. Can irritate skin and mucous membranes.
Ecological data
Molecular structure data
1. Molar refractive index: 62.51
2. Molar volume (cm3/mol): 188.0
3. Isotonic specific volume (90.2K ): 484.2
4. Surface tension (dyne/cm): 44.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 24.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 213
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. White oily liquid, slightly viscous, pure product is flaky crystals.
2. There is a faint aroma of plum and almond. Insoluble in water and glycerin, soluble in most organic solvents.
3. The chemical properties are relatively stable and can be hydrolyzed under the action of caustic alkali.
4. Exist in smoke.
5. Naturally found in Peru balsam, Tolu balsam, and essential oils such as ylang ylang oil, hyacinth oil, and tuberose oil.
Storage method
This product should be sealed and stored in a cool, dark place. Be aware of fire sources.
Synthesis method
1. Currently, alcohol exchange method is used in the fragrance industry to produce benzyl benzoate. Alcohol exchange from ethyl benzoate and benzyl alcohol.
2.The product is obtained by transesterification reaction between ethyl benzoate and benzyl alcohol, followed by water washing, layering, drying and distillation. Or it can be obtained by condensation of benzaldehyde.
Refining method: often contains impurities such as free acid and alcohol. During refining, wash with sodium bicarbonate or sodium carbonate, dry with anhydrous potassium carbonate or sodium sulfate and then distill.
3.The process reaction formula is:
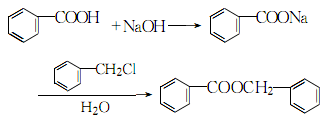
Add sodium hydroxide and water to the three-necked flask and dissolve Add benzoic acid, heat to dissolve, add benzyl chloride dropwise, and reflux at 106°C for 4 hours. Cool slightly, separate the ester layer, wash with water and alkali, and then distill under reduced pressure to obtain the finished product.
4. Use magnesium oxide as a catalyst and conduct transesterification reaction between benzyl alcohol and methyl benzoate at about 200℃.
Purpose
1. Used as a solvent and essence fixative for musk, and as a substitute for camphor in celluloid. It is also used as a plasticizer, especially when nitrocellulose is mixed with resin, it can be mixed with diethyl or dibutyl phthalate. In addition, it is also used to prepare whooping cough medicine, asthma medicine and used as insect repellent and acaricide.
2.Mainly used as a fixative. It is used to prepare fruit-based food flavors and alcoholic flavors such as strawberry, pineapple, and cherry, and is also used in daily chemical flavors such as jasmine, lily of the valley, ylang-ylang, gardenia, tuberose, and lilac. The dosage used in gum candies is 280mg/kg; in candies, it is 39mg/kg; 33mg/kg in baked goods; 45mg/kg; 12mg/kg in cold drinks.
3.Used as a solvent for cellulose acetate and cellulose nitrate. Plasticizers for plastics. Fragrance fixative.
4. It is widely used in daily chemical fragrance formulas, mainly used as a filler and flavoring agent, and is also commonly used as a diluent for many highly viscous synthetic fragrances.

 微信扫一扫打赏
微信扫一扫打赏

