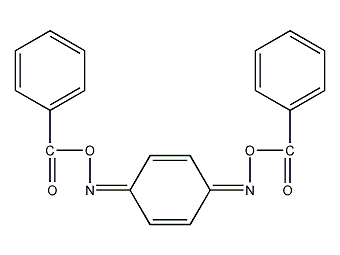
Structural formula
| Business number | 03CC |
|---|---|
| Molecular formula | C20H14N2O4 |
| Molecular weight | 346.34 |
| label |
Dibenzoyl-p-quinonedioxime, p,p’-Dibenzoylquinonedioxime, Vulcanizing agent DGM, p,p’-dibenzoylquinone dioxime, dibenzoyl-p-quinonedioxime, vulcanizing agent DGM, Vulcanizing agent |
Numbering system
CAS number:120-52-5
MDL number:None
EINECS number:204-403-4
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: purple-gray powder.
2. Density (g/mL, 20℃): 1.37
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure ( kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in chloroform, insoluble in acetone, insoluble in benzene , gasoline, ethanol and water.
20. Decomposition temperature (ºC): >200
Toxicological data
1. Acute toxicity: mouse abdominal LD50: 250mg/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 97.66
2. Molar volume (cm3/mol): 285.9
3. Isotonic specific volume (90.2K ): 751.3
4. Surface tension (dyne/cm): 47.6
5. Dielectric constant:
6.Dipole moment (10-24cm3):
7, Polarizability: 38.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 77.3
7. Number of heavy atoms: 26
8. Surface charge: 0
9. Complexity: 564
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Non-toxic.
Storage method
Storage stable.
Synthesis method
(1) Synthesis of p-quinonedioxime First add 30% sodium hydroxide solution to the reaction kettle, slowly add phenol under stirring to dissolve it in the sodium hydroxide solution. Cool the temperature to 0°C and then add sodium nitrite and 30% sulfuric acid to keep the temperature at 7 to 8°C for reaction, and nitrosophenol crystallizes and precipitates gradually. After stirring for 1 hour, let it stand for filtration, and wash the crystals with water to obtain nitrosophenol. After translocation, it is mixed with hydroxylamine hydrochloride aqueous solution and heated to 70°C for oximation reaction. After the reaction is completed, filter to obtain p-benzoquinonedioxime.
(2) Synthesis of benzoyl chloride
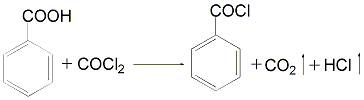
① Prepared by the reaction of benzoic acid and phosgene. Put benzoic acid into the photochemical kettle, heat and melt it, introduce phosgene at 140~150℃, and react the tail gas It contains hydrogen chloride and unreacted phosgene, which should be treated with alkali and then vented. At the end of the reaction, the temperature is lowered to -2~-3℃, followed by degassing and distillation under reduced pressure to obtain benzoyl chloride. Purity ≥98%.
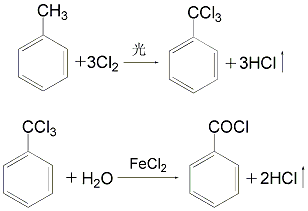
② Add toluene to the chloride kettle and illuminate it under light Chlorine gas is introduced under the reaction conditions to produce trichlorotoluene, which is then hydrolyzed in an acidic medium to produce benzoyl chloride.
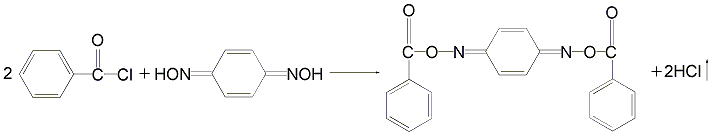
③ Dibenzoyl-p-quinone dioxime It is synthesized by the reaction of benzoyl chloride and p-quinonedioxime.
Purpose
This product is used as a vulcanizing agent for butyl rubber, natural rubber and styrene-butadiene rubber. Its performance is similar to that of p-quinonedioxime, but it has better scorch resistance. It is especially suitable for butyl rubber. In butyl rubber containing carbon black, the dosage is about 6 parts, and it is mixed with about 10 parts of lead tetroxide. In carbon black-free butyl rubber, its dosage is also about 6 parts, but the dosage of trilead tetroxide is 4 parts. This product is a very effective vulcanization aid for peroxide vulcanization, with fast vulcanization and high set elongation. This product needs to be equipped with a metal oxide activator to improve its vulcanization efficiency. It is especially suitable for butyl rubber to make inner tubes, water tires, vulcanization bladders, insulation layers of wires and cables and general rubber products.

 微信扫一扫打赏
微信扫一扫打赏

