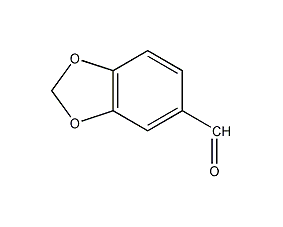
Structural formula
| Business number | 03CG |
|---|---|
| Molecular formula | C8H6O3 |
| Molecular weight | 150.13 |
| label |
jasmine aldehyde, 3,4-methylenedioxybenzaldehyde, piperonal oxide, Heliotropin, Heliotropin, 3,4-(Methylenedioxy)benzaldehyde, 1,3-Benzodioxole-5-carboxaldehyde, brightener, Synergist |
Numbering system
CAS number:120-57-0
MDL number:MFCD00005828
EINECS number:204-409-7
RTECS number:TO1575000
BRN number:131691
PubChem number:24887359
Physical property data
1. Characteristics: White crystal with the aroma of jasmine. 2. Melting point (℃): 37 3. Boiling point (℃): 263 4. Solubility: Hardly soluble in water and glycerin, easily soluble in ethanol, ether, benzyl benzoate, diethyl phthalate, soluble in Propylene glycol, 20 times soluble in olive oil.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2700mg/kg
Rat subcutaneous LD50: >5mg/kg
Rat peritoneal cavity LD50: 1500mg/kg
Mouse peritoneal cavity LC50: 480 mg/kg
Dog oral LD50: 5 mg/kg
Guinea pig oral LD50: 2 mg/kg
Rabbit oral LD50: 5mg/kg
2. Denaturation: human lymphocytes: 120ug/L; chicken embryo: 2mg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 39.16
2. Molar volume (cm3/mol): 112.2
3. Isotonic specific volume (90.2K): 307.6
4. Surface tension (dyne/cm): 56.4
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 15.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 157
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is easy to change color when the temperature is high in summer and when exposed to sunlight. dilute at room temperatureStable in acids and alkalis.
Storage method
Brown glass bottle closed light packaging. Store in a cool, dark place.
Synthesis method
1. Safrole is used as raw material to prepare jasmonaldehyde.
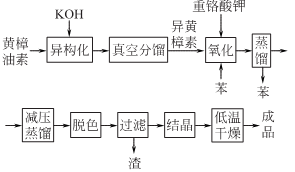
Add 6 parts of potassium hydroxide to 10 parts of alcohol and put it into the isomerization reactor. Stir and heat to reflux to dissolve the potassium hydroxide. Then add 6 parts of pure safrole to this solution, slowly heat the mixture to reflux, and react for 15 to 20 hours. After the reaction is complete, cool the mixture, dilute it with water, and then evaporate most of thealcohol. The remaining crude isosafrole is removed from impurities by vacuum fractionation. In the glass-lined reaction kettle, first put 26 parts of distilled water, 11.6 parts of sodium dichromate, and a small amount of p-aminobenzoic acid, and stir to dissolve. Add 6.6 parts of isosafrole, stir and mix at room temperature, and slowly add 98% industrial sulfuric acid while stirring. Because the reaction is exothermic, cold water must be passed through the jacket for cooling, and sulfuric acid must be added slowly. After adding sulfuric acid, continue the reaction for 0.5h and then let it stand for layering. The benzene layer is separated, and the remaining acidic mixture is extracted again with 8 parts of benzene at 40°C. Combine the benzene liquid extracted twice, wash and neutralize it, and then steam out (recycle) the benzene. The crude jasmonaldehyde left in the distillation kettle is then subjected to vacuum fractionation to obtain semi-solid jasmonaldehyde. Dissolve it in an equal amount of 95% alcohol, heat it slightly to completely dissolve the jasmonaldehyde, add an appropriate amount of activated carbon and stir for 0.5 hours, then filter, cool, crystallize and dry at low temperature to obtain jasmonaldehyde.
2. Tobacco: BU, 14; synthesis: semi-synthetic and fully synthetic. The semi-synthetic method uses safrole in natural sassafras oil as raw material, isomerizes it into isosafrole under alkaline conditions, and then oxidizes it with ozone to form jasmonaldehyde. The total synthesis method uses catechol and glyoxylic acid as raw materials.
Purpose
1. Can be used as raw material for the preparation of edible flavors (GB 2760-86)
2. Used for the preparation of vanilla, peach, strawberry, cherry Food flavors, etc. are also used in cola, wine and tobacco. The dosage used in chewing gum is 36mg/kg; in baked goods it is 18mg/kg; Candy 7.4mg/kg; Cold drinks 7mg/kg; In soft drinks, 6.0mg/kg; In puddings, 55.8mg/kg.
3. It is widely used in food flavors, tobacco flavors, soaps and daily cosmetics flavors. It is not only the main blending agent of jasmine flower fragrance, but also a fixative. It is used in many floral essences such as sunflower, lily, and violet. It is also used as a food pesticide and a brightener in the electroplating industry.

 微信扫一扫打赏
微信扫一扫打赏

