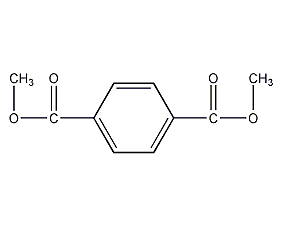
Structural formula
| Business number | 03CJ |
|---|---|
| Molecular formula | C10H10O4 |
| Molecular weight | 194.18 |
| label |
1,4-Benzenedicarboxylic acid dimethyl ester, dimethyl phthalate, dimethyl terephthalate, dimethyl terephthalate, Dimethyl 1,4-phthalate, dimethyl terephthalate, 1,4-Benzenedicarboxylic acid dimethyl ester, Dimethyl 1,4-benzenedicarboxylate, resin solvent, Toughening agent |
Numbering system
CAS number:120-61-6
MDL number:MFCD00008440
EINECS number:204-411-8
RTECS number:WZ1225000
BRN number:1107185
PubChem number:24862794
Physical property data
1. Properties: White needle-like crystals, easy to sublimate.
2. Boiling point (ºC, 101.3kPa): 288
3. Melting point (ºC): 141
4. Relative density (g/mL, 150 /4ºC): 1.084
5. Refractive index (150ºC): 1.4752
6. Viscosity (mPa·s,150ºC): 0.965
7. Flash Point (ºC, opening): 146~147
8. Ignition point (ºC): 570
9. Critical temperature (ºC): 489
10. Critical pressure (MPa): 2.7
11. Vapor pressure (kPa, 148ºC): 1.3
12. Solubility: soluble in methanol, ether, acetone, chloroform, with temperature As it rises, the solubility increases and it becomes insoluble in water.
13. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -4631.6
14. The standard claim heat of the crystal phase ( Enthalpy) (kJ·mol-1): -732.6
Toxicological data
1. Acute toxicity: Rat passage LD50:> 3200mg/Kg; Rat peritoneal cavity ld50: 3900mg/kg
Guinea and Mouse through subcutaneous LD50: 5g/kg; Rabbit intravenous injection LD50: 300mg/kg
2. Other multiple dose toxicity: oral TDLO in rats: 57600mg/kg/14W-C; inhaled TDLO in rats: 1mg/m3/2H/ 22W-I
3. Mutagenicity: mouse micronucleus test: 200umoL/kg
4. Low toxicity, not absorbed through the skin, and non-irritating to the skin.
Ecological data
None yet
Molecular structure data
1��� Molar refractive index: 49.79
2. Molar volume (cm3/mol): 165.2
3. Isotonic specific volume (90.2K): 416.9
4. Surface tension (dyne/cm): 40.5
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 19.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 192
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless orthorhombic crystal. Viscosity (150℃) 0.965mPa·s, ignition point 155℃. Soluble in hot ethanol, methanol, ether, chloroform, insoluble in water. Vapor or dust can form explosive mixtures with air, with a minimum explosion limit of 0.03% (volume).
Chemical properties: transesterification and polycondensation reactions, hydrogenation and hydrogenolysis reactions and hydrolysis reactions can occur.
2.This product has very low toxicity and no skin irritation. The oral LD50 of rats is 10g/kg.
Storage method
Seal and save. Packed in plastic woven bags. Store in a cool and ventilated place.
Synthesis method
Obtained from the esterification of terephthalic acid and methanol. The earliest sulfuric acid catalyst was used, the reaction temperature was 65-100°C, and the esterification time was 10-16 hours. This method consumes a lot of methanol and has low production capacity. By increasing the reaction pressure and esterifying at 0.39-0.49MPa, the reaction time can be shortened to 1-2h, and the loss of methanol is also reduced compared to the normal pressure method. In recent years, a high-temperature and high-pressure liquid phase esterification method has been adopted. The reaction temperature is 250-300°C and the pressure is 2-2.5MPa. Tin, zinc, and antimony compounds can be used as catalysts, or no catalyst can be used. The yield based on terephthalic acid can reach 96-98% or higher. Another way to produce DMT is through the Witten-Hercules process, which uses the staged oxidative esterification of paraxylene. This method uses two methyl oxidation processes of paraxylene. , methyl esterify the first oxidized carboxyl group to avoid side reactions in the next step of oxidation. In order to simplify the process, the two-step oxidation can be combined in one oxidation reactor, and the two-step esterification can also be combined in one esterification reactor. The raw material paraxylene and the recycled methyl paratoluate are simultaneously oxidized in a tower reactor with a reaction temperature of 140-170°C and a pressure of 0.4-0.7MPa. Cobalt salt or cobalt and manganese salts are used as catalysts. Air is continuously oxidized to produce p-toluic acid and monomethyl terephthalate. The oxidation product undergoes esterification reaction with excess methanol at 200-280°C and 2-2.5MPa. Methanol is distilled from the top of the esterification reactor and recycled after distillation; the crude ester from the bottom of the esterification reactor is distilled to separate methyl p-toluate (circulated to the oxidation reactor) and crude p-benzene. Dimethyl dicarboxylate. Dissolve the crude dimethyl terephthalate with methanol in the dissolver, and then send it to the crystallizer to precipitate the crystals. Centrifuge the obtained dimethyl terephthalate and then distill and purify it to obtain a fiber-grade product with purity ≥99.9%. Raw material consumption quota: paraxylene 630kg/t, methanol 440kg/t. In the industrial production of dimethyl terephthalate, the segmented oxidative esterification method of paraxylene has developed rapidly. This method does not require a solvent during the oxidation reaction, does not require titanium in the reactor, and the product is easy to purify. Therefore, it was adopted by many countries after the 1950s. The disadvantage is the low overall yield in terms of paraxylene (87%).
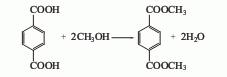
Purpose
Dimethyl terephthalate is mainly used to make polyester resin, which in turn makes films and fibers, as well as high-strength polyester insulating paint. Polyester film is mainly used as electrical insulation material and film production (such as as the base material for movie films, X-ray films, photographic films, audio tapes, video tapes, computer tapes, etc.). The most common use of polyester resin is to make fibers for fabrics, fishing nets, carpets, tire cords, etc. It can also be used to produce the plasticizer dioctyl terephthalate. Resin solvent, toughening agent, organic synthesis intermediate.
Mainly used as an intermediate for polyester resin and the manufacture of polyester resin. It is also used in the production of insulating paint and adhesives. It can also be used to produce the plasticizer dioctyl terephthalate.

 微信扫一扫打赏
微信扫一扫打赏

