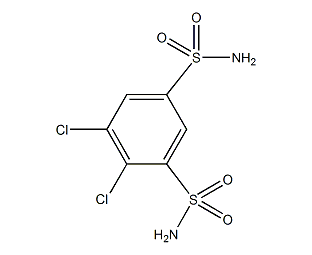
Structural formula
| Business number | 03D3 |
|---|---|
| Molecular formula | C6H6Cl2N2O4S2 |
| Molecular weight | 305.16 |
| label |
4,5-dichlorobenzene-1,3-disulfonamide, Dichlorosulfonamide, dichlorphenamide, Bischlorosulfonamide, 1,3-Benzenedisulfonamide, 4,5-dichloro-, 1,3-disulfamoyl-4,5-dichlorobenzene, 1,3-Disulfamyl-4,5-dichlorobenzene, 3,4-Dichloro-5-sulfamylbenzenesulfonamide, 4,5-Dichloro-1,3-benzenedisulfonamide, 4,5-Dichloro-1,3-disulfamoylbenzene, 4,5-dichloro-3-benzenedisulf, Heterocyclic compounds |
Numbering system
CAS number:120-97-8
MDL number:MFCD00148948
EINECS number:204-440-6
RTECS number:CZ9200000
BRN number:None
PubChem ID:None
Physical property data
1. Character:White or nearly white crystalline powder
2. Melting point (℃):239~241
Toxicological data
1, acute toxicity data: Rat oral LD50: 10070mg/kg
Rat peritoneal cavityLD50:1244mg/kg
Mouse oral LD50: 1710mg/kg
Mouse peritoneal cavityLD50:304mg/kg
small Mouse intravenous injectionLD50:643mg/kg
Dog intravenous injection LD50: 200mg/kg
2, reproductive toxicity data: Rat (female) oral TDLO: 4642mg/kg/1-22D
Mouse(女)经Peritoneal cavityTDLO:500mg/kg/10D
Mouse(Female) Subcutaneous TDLO: 96mg/kg/15D
Mouse(Female) Gastrointestinal TDLO: 144mg/kg/13D
Ecological data
None
Molecular structure data
5. Molecular property data: 1. Molar refractive index: 61.28 2. Molar volume(m3/ mol):171.2 3. isotonic ratio(90.2K):494.9 Mouse(female) subcutaneously TDLO: 96mg/kg/15D Mouse(Female) Gastrointestinal TDLO: 144mg/kg/13D
Ecological data
None
Molecular structure data
5. Molecular property data: 1. Molar refractive index: 61.28 2. Molar volume(m3/ mol):171.2 3. isotonic ratio(90.2K):494.9 4. Surface Tension(dyne/cm):69.7 5. Dielectric constant: 6. Dipole moment(10 -24cm3): 7. Polarizability: 24.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 137
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 452
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
White or nearly white crystalline powder. Melting point 239-241℃. Soluble in ethanol, easily soluble in alkaline solution, almost insoluble in water or chloroform. Odorless, slightly bitter taste.
Storage method
None
Synthesis method
2. Brief description of production method
Obtained from chlorosulfonation and amination of o-dichlorobenzenePurpose
3. Purpose
This product is an inhibitor of carbonic anhydrase and has the effects of lowering intraocular pressure and diuresis. However, the diuretic effect is very weak and is mainly used to treat glaucoma to reduce intraocular pressure
p-alt: auto; mso-margin-bottom-alt: auto” align=left>4. Surface Tension(dyne/cm):69.7
5. Dielectric constant:
6. Dipole moment(10 -24cm3):
7. Polarizability: 24.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 137
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 452
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
White or nearly white crystalline powder. Melting point 239-241℃. Soluble in ethanol, easily soluble in alkaline solution, almost insoluble in water or chloroform. Odorless, slightly bitter taste.
Storage method
None
Synthesis method
2. Brief description of production method
Obtained from chlorosulfonation and amination of o-dichlorobenzenePurpose
3. Purpose
This product is an inhibitor of carbonic anhydrase and has the effects of lowering intraocular pressure and diuresis. However, the diuretic effect is very weak and is mainly used to treat glaucoma to reduce intraocular pressure
�It is an inhibitor of carbonic anhydrase and has the effects of reducing intraocular pressure and diuresis. However, the diuretic effect is very weak and is mainly used to treat glaucoma to reduce intraocular pressure

 微信扫一扫打赏
微信扫一扫打赏

