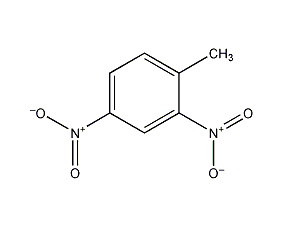
Structural formula
| Business number | 03D9 |
|---|---|
| Molecular formula | C7H6N2O4 |
| Molecular weight | 182.13 |
| label |
1-Methyl-2,4-dinitrobenzene, 2,4-dinitrotoluene, 2,4-dinitrobenzene, 2,4-Dinitrate [grass (above) + nao], diniter [grass (above) + nao], Dinitoluene, 1-methyl-2,4-dinitro-benzen, 2,4-dinitro-toluen, 4-methyl-1,3-dinitrobenzene, Benzene,1-methyl-2,4-dinitro-, dinitrotoluene(non-specificname), dinitrotoluenes,molten, Dinitrotoluol, NCI-C01865, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:121-14-2
MDL number:MFCD00007172
EINECS number:204-450-0
RTECS number:XT1575000
BRN number:1912834
PubChem number:24869585
Physical property data
1. Characteristics: light yellow needle-like crystals with a bitter almond flavor. [1]
2. Melting point (℃): 69.5~71[2]
3. Boiling point (℃) : 300 (decomposition) [3]
4. Relative density (water = 1): 1.32[4]
5. Relative vapor density (air = 1): 6.27[5]
6. Saturated vapor pressure (kPa): 13.33 (157.7℃)[6]
7. Heat of combustion (kJ/mol): -3564.7[7]
8. Octanol/water partition coefficient: 1.98[8]
9. Flash point (℃): 206.7 (CC) [9]
10. Ignition temperature (℃): 360[10]
11. Solubility: Slightly soluble in water, ethanol, ether, easily soluble in benzene and acetone. [11]
Toxicological data
1. Acute toxicity[12] LD50: 268mg/kg (rat oral); >1g/kg (guinea pig oral Skin)
2. Irritation[13] Rabbit transdermal: 500mg (24h), mild irritation .
3. Mutagenicity[14] Microbial mutagenicity: Salmonella typhimurium 10μg/dish. Sex chromosome deletion and non-disjunction: Drosophila melanogaster parenteral 200ppm. DNA damage: rat liver 3mmol/L. Unprogrammed DNA synthesis: Rat oral administration 35 mg/kg.
4. Carcinogenicity[15] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
5. Others[16] The lowest oral toxic dose in rats (TDLo): 1050mg/kg (pregnant 7~20d), causingAbnormal development of the fluid and lymphatic systems (including spleen and bone marrow) and delayed effects (neonatal rats).
Ecological data
1. Ecotoxicity[17]
LC50: 23~25.6mg/L (96h) ( Fathead minnow)
IC50: 0.13~2.7mg/L (72h) (algae)
2. Biodegradability[18]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 48~240
3. Non-biodegradability[19]
Aqueous phase photolysis half-life (h): 23~72
Photooxidation half-life in water (h): 3~33
Photooxidation half-life in air (h): 284~2840
4. Bioconcentration[20] BCF: 204 (guppy)
Molecular structure data
1. Molar refractive index: 44.16
2. Molar volume (cm3/mol): 129.3
3. Isotonic specific volume (90.2K ): 355.8
4. Surface tension (dyne/cm): 57.2
5. Polarizability (10-24cm3): 17.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 91.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 220
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is toxic. Cats will not be poisoned if they inhale 21×10-6 vapor for 7 hours, but inhalation through the skin can cause poisoning. For people, it causes headaches, dizziness, and sometimes mild cyanosis. However, after drinking a large amount of alcohol at the same time, they will suddenly become unconscious and comatose and fall ill within a few hours. The maximum allowable concentration of dinitrotoluene in the air in the workplace is 1.5×10-6. Workplace equipment must be sealed to prevent escape, leakage, dripping and leakage. Operators should wear protective equipment and avoid direct contact. It is strictly prohibited to drink alcohol before or after work.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidizing agent, strong reducing agent, strong alkali
4. Conditions to avoid contact [23] Heat and friction , vibration or impact
5. Polymerization hazard[24] No polymerization
6. Decomposition products[25] Nitrogen oxides
Storage method
Storage Precautions[26] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 35°C and the relative humidity does not exceed 80%. The packaging is sealed. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the nitration of p-nitrotoluene: Add the nitrification waste acid (sulfuric acid concentration is 77%) into the nitrification kettle, then add the melted p-nitrotoluene and stir to lower the temperature in the kettle to about 55°C . Then add the mixed acid (composed of 31.75% nitric acid and 64.85% sulfuric acid) in two batches. The first batch of feeding temperature is 52-56℃. The second batch of feeding process temperature gradually rises from 54℃ to 70℃, and the feeding process takes about 6-7 hours. At the end, the reaction was continued at 75°C for nearly 3 hours. After the reaction is completed, add cold water to dilute it with stirring and let it cool. After all the reactants in the upper layer have solidified, release the waste acid (part of it is applied). Heat the reactant to melt again, stir it slightly and let it cool. After the reactant solidifies, put the waste acid again. The reactants are melted and neutralized with liquid caustic soda at 68-70°C until the pH value is 5-6. Spray in cold water to cool down, cool to 35°C, filter, and wash with water to obtain 2,4-dinitrotoluene. Raw material consumption quota: p-nitrotoluene 774kg/t, sulfuric acid (95.5%) 785kg/t, nitric acid (98%) 362kg/t.
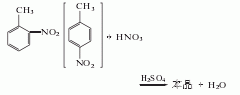
2. Obtained from nitration of toluene : First, crude nitrotoluene is obtained with a yield of 97%, containing 62-63% o-nitrotoluene, 33-34% p-nitrotoluene and 3-4% m-nitrotoluene. Add mixed acid dropwise to this crude nitrotoluene. Stirring and nitration reaction yielded crude dinitrotoluene (CAS number [25321-14-6], a mixture of three isomers) with a yield of 98.6%. The general situation of material balance in the nitrification process is as follows. The dinitrotoluene mixture is dissolved in methanol or ethanol, and then cooled to crystallize 2,4-dinitrotoluene, separated, and distilled to obtain 2,4-dinitrotoluene. The former method is mainly used to manufacture pure 2,4-dinitrotoluene, that is, the nitration method of p-nitrotoluene. The purity of industrial products is ≥95%.
Purpose
1. This product can be derived from a series of intermediates, for example: these intermediates are used to produce sulfide yellow GC, sulfide yellow brown 5G, sulfide yellow brown 6G, sulfide red brown B3R and other dyes, to produce explosives TNT, and to produce 2, 4-Toluene diisocyanate. Toluene diisocyanate in these intermediates is used in large quantities and is mainly used in bulk products such as polyurethane foam, polyurethane elasticity, polyurethane coatings, etc.
2.DNT is an important explosive and a raw material for the preparation of 2,4-dinitrotoluene. It can also be used in organic synthesis industries such as polyurethane, dyes, medicine, and rubber.
3. Dye intermediates, mainly used to prepare azo dyes. It is also a raw material for organic synthesis. 2,4-diaminotoluene, aminotoluene, etc. can be obtained through reduction.
4. Used in the manufacture of dye intermediates, explosives, and organic synthesis. [27]
Materials, production of explosives TNT, production of 2,4-toluene diisocyanate. Toluene diisocyanate in these intermediates is used in large quantities and is mainly used in bulk products such as polyurethane foam, polyurethane elasticity, polyurethane coatings, etc.
2.DNT is an important explosive and a raw material for the preparation of 2,4-dinitrotoluene. It can also be used in organic synthesis industries such as polyurethane, dyes, medicine, and rubber.
3. Dye intermediates, mainly used to prepare azo dyes. It is also a raw material for organic synthesis. 2,4-diaminotoluene, aminotoluene, etc. can be obtained through reduction.
4. Used in the manufacture of dye intermediates, explosives, and organic synthesis. [27]

 微信扫一扫打赏
微信扫一扫打赏

