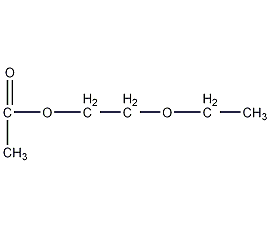
Structural formula
| Business number | 032U |
|---|---|
| Molecular formula | C6H12O3 |
| Molecular weight | 132.16 |
| label |
2-Ethyldioxyacetate, 2-ethoxyethyl acetate, Glycol ether acetate, Ethyl Cellosolve Acetate, EGEA, 2-Ethoxyethyl acetate, Acetic acid glycol ether, Acid ethyl cellosolve, Monoethyl glycol acetate, leather adhesive, paint stripper, Thinner for adhesives, Solvents for water-soluble paints |
Numbering system
CAS number:111-15-9
MDL number:MFCD00009251
EINECS number:203-839-2
RTECS number:KK8225000
BRN number:1748677
PubChem number:24845082
Physical property data
1. Properties: colorless liquid with a faint aromatic odor. [1]
2. Melting point (℃): -61.7[2]
3. Boiling point (℃): 156.4[3]
4. Relative density (water=1): 0.97 (20℃)[4]
5. Relative vapor density (air = 1): 4.72[5]
6. Saturated vapor pressure (kPa): 0.27 (20℃)[6]
7. Heat of combustion (kJ/mol): -3304.5[7]
8. Critical temperature (℃): 334[8]
9. Critical pressure (MPa): 3.0[9]
10. Octanol/water partition coefficient: 0.65[10]
11. Flash point (℃): 47 (OC); 52 (CC) [11]
12. Ignition temperature (℃): 379[12]
13. Explosion upper limit (%): 14[13]
14. Lower explosion limit (%): 1.7[14]
15. Solubility: slightly soluble in water, soluble in ethanol and ether, miscible In most organic solvents such as aromatics. [15]
16. Refractive index (25ºC): 1.4023
17. Refractive index (30ºC): 1.4003
18 .Viscosity (mPa·s, 25ºC): 1.025
19. Flash point (ºC, closed): 51
20. Flash point (ºC, open): 66
21. Vapor pressure (kPa, 20ºC): 0.15
22. Vapor pressure (kPa, 48ºC): 1.33
23. Vapor pressure (kPa, 81ºC): 6.67
24. Heat of evaporation (KJ/mol): 44.4
25. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.07
26. Conductivity (S/m): 2×10-8
27. Volume expansion coefficient (K-1,10~ 30ºC): 0.00111
Toxicological data
1. Acute toxicity[16]
LD50: 2900mg/kg (rat oral); 10500μl (10185mg)/kg ( Rabbit transdermal)
LC50: 12100mg/m3 (Rat inhalation, 8h)
2. StingIrritation[17]
Rabbit transdermal: 490 mg, mild stimulation (open stimulation test).
Rabbit eye: 40mg, moderate irritation.
Ecological data
1. Ecotoxicity[18] LC50: 43.5mg/L (48h), 42.2mg/L (96h) (fathead minnow)
2. Biodegradability [19] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 86.9% degradation after 2 weeks.
3. Non-biodegradability[20] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 1.5d (theoretical).
When the pH value is 7 and 8, the hydrolysis half-life is 305d and 30d respectively (theoretical).
Molecular structure data
1. Molar refractive index: 33.36
2. Molar volume (cm3/mol): 137.4
3. Isotonic specific volume (90.2K ): 315.2
4. Surface tension (dyne/cm): 27.6
5. Polarizability (10-24cm3): 13.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 80.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Miscible with general organic solvents and soluble in water. Has a pleasant ester scent. As a new generation of universal solvent, it has extremely strong dissolving ability, especially for polymers. Has the properties of aliphatic ether and acetate.
2. Stability[21] Stable
3. Incompatible substances[22] Acids, alkalis, strong oxidants
4. Polymerization hazard[23] No polymerization
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of ethylene glycol monoethyl ether and acetic anhydride. Mix acetic anhydride and concentrated sulfuric acid. After heating to 130°C, slowly add ethylene glycol monoethyl ether dropwise. The reaction temperature is maintained at 130-135°C. After adding, flow for another 1-2 hours, and the reflux temperature is 140°C. After cooling, neutralize with sodium carbonate to pH=7-8, and then dry with industrial anhydrous potassium carbonate. Filter out the desiccant for crude fractionation, and collect the distillate between 150-160°C. Fractionate again and collect the fraction at 155.5-156.5°C as the finished product.

It can also be made of ethylene glycol monoethyl ether Catalyzed with acetic acid and concentrated sulfuric acid, it is obtained by refluxing in benzene. Ethylene glycol monoethyl ether reacts with acetic acid under the catalysis of cationic resin, and the generated water is continuously removed, and then the finished product is obtained by distillation. The acetic acid conversion rate of this method is 94.5%, and the product selectivity is 98.5%.

2, 2-ethoxy Acetic acid and ethanol reaction method.
3. Ethyl acetate and ethoxyethanol transesterification method.
4. Reaction method of acetic acid and ethoxyethanol.
5. Reaction method of ethylene oxide and ethyl acetate.
6. Heterogeneous catalytic ethylene oxide method This process uses highly active solid acid as a catalyst, and directly uses ethylene oxide and acetic acid at normal temperature (50~100℃) and normal pressure. Ethyl ether is used as raw material to synthesize ethylene glycol ether acetate in the next step under heterogeneous conditions. The process steps are simple, the investment in production equipment is low, the catalyst is pollution-free, and it has strong technical advantages. It is the latest method developed by Fushun Chemical Industry Research and Design Institute. Under the synthesis reaction: 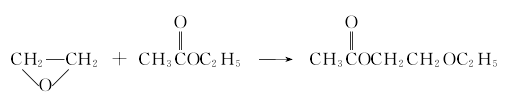
Purpose
1. This product can dissolve rosin resin, nitrocellulose, ethylcellulose, polyvinyl chloride, polystyrene, polymethyl methacrylate, polyvinyl acetate, phenolic resin, alkyd resin, etc. Used as a solvent for metal, furniture spray paint and other coatings and inks. It is also used as a diluent for adhesives and a solvent for water-soluble paints. Used in combination with other compounds as leather adhesive; paint stripper; metal hot-dip anti-corrosion coating, etc.
2. Has many special uses. Soluble rosin resin, polystyrene, polyvinyl acetate, polyvinyl chloride, polypervinyl chloride, polyurethane, epoxy resin, nitrocellulose, ethylcellulose, polymethylmethacrylate, phenolic resin, alkyd Resin, natural rubber, chloroprene rubber, chlorinated rubber, ethylene-propylene rubber, etc. It is also used as a diluent for adhesives and a solvent for water-soluble paints. It can also be used as a solvent for metal, furniture spray paint and other coatings and inks.
3. Used as a solvent for nitrocellulose, grease, resin, and paint remover. [25]
Rubber, chloroprene rubber, chlorinated rubber, ethylene-propylene rubber, etc. It is also used as a diluent for adhesives and a solvent for water-soluble paints. It can also be used as a solvent for metal, furniture spray paint and other coatings and inks.
3. Used as a solvent for nitrocellulose, grease, resin, and paint remover. [25]

 微信扫一扫打赏
微信扫一扫打赏

