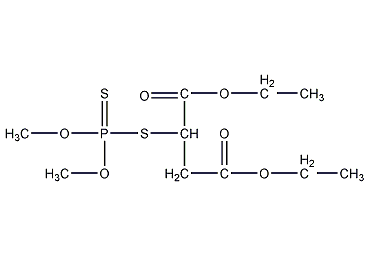
Structural formula
| Business number | 03E1 |
|---|---|
| Molecular formula | C10H19O6PS |
| Molecular weight | 330.36 |
| label |
marathon, Marathon, insecticide phosphorus, Liangchongjing, 1,2-Bis(ethoxyformyl)ethyl-O,O-dimethylphosphorodithioate, Fosfothion, Fyfanon, Karbofos, Malafor, 1,2-Bis(ethoxycarbonyl)ethyl-O,O-dimethyl phosphorodithioate, Organophosphorus pesticides |
Numbering system
CAS number:121-75-5
MDL number:MFCD00036233
EINECS number:204-497-7
RTECS number:WM8400000
BRN number:1804525
PubChem number:24862222
Physical property data
1. Properties: colorless to light yellow oily liquid with garlic odor, industrial product with dark brown color. [1]
2. Melting point (℃): 2.9~3.0[2]
3. Boiling point (℃) : 156~157 (0.09kPa) [3]
4. Relative density (water = 1): 1.23 (25℃) [4]
5. Saturated vapor pressure (kPa): 1.43 (156℃)[][5]
6. Pungent Alcohol/water partition coefficient: 2.36[6]
7. Flash point (℃): >162 (OC); 163 (CC) [7]
8. Solubility: Slightly soluble in water, easily soluble in most organic solvents such as alcohol, ether, and ketone. [8]
Toxicological data
1. Acute toxicity[9]
LD50: 1800mg/kg (rat oral)
LC50: 84.6mg/m3 (rat inhalation, 4h)
2. Irritation No information available p>
3. Subacute and chronic toxicity[10] The chronic toxicity is very low. Rats are fed with feed containing 5000ppm of this product. 2a, no death occurs.
4. Mutagenicity[11] Microbial mutagenicity: Salmonella typhimurium 10mg/L; Bacillus subtilis 1nmol/dish; other microorganisms 100mg/L. Sister chromatid exchange: human lymphocytes 20mg/L; human fibroblasts 5mg/L.
5. Teratogenicity[12] 91 days before mating to 1 to 20 days after pregnancy in rats, oral administration The lowest toxic dose (TDLo) of 5550 mg/kg caused body wall development malformations. The lowest toxic dose (TDLo) of 283 mg/kg was administered orally to rats on the 9th day after pregnancy, which caused developmental malformations in the genitourinary system.
6. Carcinogenicity[13] IARC Carcinogenicity Comment: Group 3, available evidence is not conclusive for humans Carcinogenicity classification.
7. Others[14] The lowest oral toxic dose in rats (TDLo): 5550mg/kg (pregnant 91d/1~20d),��Abnormal body wall development. The lowest oral toxic dose in rats (TDLo): 283 mg/kg (9 days of pregnancy), genitourinary system abnormalities.
Ecological data
1. Ecotoxicity[15]
LC50: 0.027~3.2mg/L (96h) ( Fish)
EC50: 0.0018mg/L (48h) (Daphnia)
2. Biodegradability[16]
Aerobic biodegradation (h): 100~1236
Anaerobic biodegradation (h): 400~4944
3. Non-biodegradability[17]
Aqueous phase photolysis half-life (h): 990~20000
Photooxidation half-life in air (h): 1~9.8
First-level hydrolysis half-life (h): 0.00077
Molecular structure data
1. Molar refractive index: 77.50
2. Molar volume (cm3/mol): 259.6
3. Isotonic specific volume (90.2K ): 680.4
4. Surface tension (dyne/cm): 47.1
5. Polarizability: 30.72
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 8
4. Number of rotatable chemical bonds: 11
5. Number of tautomers: none
6. Topological molecule polar surface area 128
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 341
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances [19] Strong oxidants, alkalis
3. Conditions to avoid contact[20] Heating
4. Polymerization hazard[21] No polymerization p>
5. Decomposition products[22] Phosphorus oxide, sulfur oxide
Storage method
Storage Precautions[23] Stored in a cool, well-ventilated special warehouse, implementing the “two people to send and receive, two people to keep” system . Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Dimethylphosphorodithioate is synthesized from phosphorus pentasulfide and methanol, and then added with diethyl maleate to obtain malathion. Put diethyl maleate (380kg based on 100%) into a 1000L glass-lined reaction pot and stir, control the temperature below 45°C, and put in 350kg phosphorus pentasulfide. Slowly add methanol dropwise, control the temperature to 48-55°C, and finish adding 250kg of methanol in about 2 hours. After the addition is completed, keep the reaction at 65-75°C for 8 hours. The reaction produces hydrogen sulfide and escapes methanol vapor. The methanol is refluxed through the condenser, and the hydrogen sulfide is absorbed by alkali liquid. This process is carried out under negative pressure. After the reaction is completed, cool to 40-50°C and let stand for layering to precipitate impurities. The upper crude oil is washed with water, alkali and water, and then dehydrated in a vacuum dehydration pot. The water in the crude oil is evaporated below 85°C (vacuum degree 93.3kPa) for 1.5-2 hours to obtain malathion crude oil. Raw material consumption quota: maleic anhydride 430kg/t, phosphorus pentasulfide 470kg/t, ethanol 420kg/t, methanol 390kg/t.
Purpose
1. Malathion is a highly efficient and low-toxic insecticide and acaricide with a wide range of prevention and control. It is not only used for rice, wheat, and cotton, but also used for insect control on vegetables, fruit trees, tea, and warehouses due to its low toxicity and short residual effect. Mainly used to control rice planthoppers, rice leafhoppers, cotton aphids, cotton red spider mites, wheat armyworms, pea weevils, soybean heartworms, fruit tree spider mites, aphids, mealybugs, nest moths, vegetable yellow streaks, leaf insects, Various scales on tea trees, as well as mosquitoes, fly larvae and bed bugs.
2. Used as pesticide and insecticide. [24]

 微信扫一扫打赏
微信扫一扫打赏

