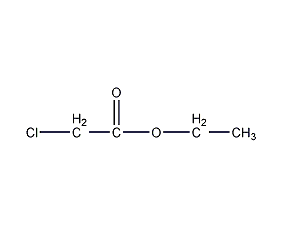
Structural formula
| Business number | 02RM |
|---|---|
| Molecular formula | C4H7ClO2 |
| Molecular weight | 122 |
| label |
Ethyl chloroacetate, Chloroacetic Acid Ethyl Ester, Pesticide intermediates; aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:105-39-5
MDL number:MFCD00000932
EINECS number:203-294-0
RTECS number:AF9110000
BRN number:506455
PubChem number:24854517
Physical property data
1. Properties: colorless liquid with pungent smell[1]
2. Melting point (℃): -26[2]
3. Boiling point (℃): 143~146[3]
4. Relative density (water=1): 1.16[ 4]
5. Relative vapor density (air=1): 4.3~4.46[]5
6. Saturated vapor pressure ( kPa): 1.33 (38℃)[]6
7. Critical pressure (MPa): 3.79[7]
8. Octanol/water partition coefficient: 0.94[8]
9. Flash point (℃): 64 (OC)[9]
10. Solubility: Insoluble in water, miscible in ethanol, ether and benzene. [10]
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit eye contact, 250μg/24HREACTION SEVERITY, strong reaction; Standard Dresser test: rabbit eye contact, 250μg/24HREACTION SEVERITY, strong reaction;
2. Acute toxicity: rat oral LD50: 235mg/kg; mouse oral LD50: 350mg/kg; mouse subcutaneous LD50: 250mg/kg; rabbit skin contact LD50: 230mg/kg;
3. Chronic toxicity/carcinogenicity: mouse peritoneal cavity TDLo: 2940mg/kg/8W-I;
4. It has irritating effects on eyes, skin and mucous membranes, and its vapor has anesthetic properties.
5. Acute toxicity[11]
LD50: 50mg/kg (rat Oral); 230mg/kg (rabbit transdermal)
6. Irritation[12] Rabbit Eye: 250μg, severe irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[13] When the pH value is 7 and 8, the hydrolysis half-life is 9d and 22h respectively (theoretical)
Molecular structure data
1. Molar refractive index: 27.20
2. Molar volume (cm3/mol): 109.4
3. Isotonic specific volume (90.2K ): 254.4
4. Surface tension (dyne/cm): 29.2
5. Polarizability: 10.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 62.7
10. IsotopesNumber of �� atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[14] Stable
2. Incompatible substances[15] Acids, alkalis, strong oxidants, strong reducing agents
3. Polymerization hazard[16] No polymerization
4. Decomposition products[17] Hydrogen chloride
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the esterification of chloroacetic acid and ethanol. Chloroacetic acid and ethanol are directly esterified using sulfuric acid as a catalyst. The crude ester is neutralized by alkali washing, water washing and distillation to obtain the finished product.
![]()
Purpose
Used as solvent for organic synthesis. [19]

 微信扫一扫打赏
微信扫一扫打赏

