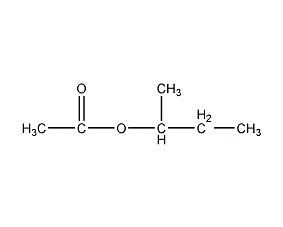
Structural formula
| Business number | 02RR |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
sec-butyl acetate, Acetic Acid sec-Butyl Ester, paint solvents, thinner, Gasoline antiknock agent |
Numbering system
CAS number:105-46-4
MDL number:MFCD00009328
EINECS number:203-300-1
RTECS number:AF7380000
BRN number:1720689
PubChem number:24854570
Physical property data
1. Properties: colorless liquid with fruity aroma. [1]
2. Melting point (℃): -98.9[2]
3. Boiling point (℃): 112.3[3]
4. Relative density (water = 1): 0.86[4]
5. Relative vapor Density (air=1): 4.00[5]
6. Saturated vapor pressure (kPa): 1.33 (20)[6]
7. Heat of combustion (kJ/mol): -3556.3[7]
8. Critical temperature (℃): 288[8]
9. Critical pressure (MPa): 3.24[9]
10. Octanol/water partition coefficient: 1.72[ 10]
11. Flash point (℃): 31 (OC); 16.7 (CC) [11]
12. Ignition temperature (℃): 421[12]
13. Explosion limit (%): 9.8[13]
14. Lower explosion limit (%): 1.7[14]
15. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol and ether. [15]
16. Flash point (ºC, closed): 19
17. Flash point (ºC, open): 31.1
18. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 1.92
19. Liquid phase standard hot melt (J·mol-1· K-1): 230.6
20. Volume expansion coefficient (K-1, 10~30ºC): 0.00118
21. Eccentricity factor: 0.406
Toxicological data
1. Acute toxicity[16] LD50: 3200mg/kg (rat oral)
2. Irritation No information yet
3. Others[17] LCLo: 24000ppm (rat inhalation, 4h)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 70h (theoretical).
When the pH value is 7, 8, and 9, the hydrolysis half-life is 12.6a, 1.26a, and 46d respectively (theoretical).
4. Other harmful effects [19] This substance may be harmful to the environment, so special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 31.57
2. Molar volume (cm3/mol): 131.4
3. Isotonic specific volume (90.2K ): 292.9
4. Surface tension (dyne/cm��: 24.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 12.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 78.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: similar to butyl acetate. When heated to 500°C, it decomposes into 1-butene, 2-butene, ethylene and propylene. When sec-butyl acetate is passed through glass wool at 460~473°C in nitrogen flow, 56% 1-butene, 43% 2-butene and 1% propylene are generated. When heated to 380°C in the presence of thorium oxide, it decomposes into hydrogen, carbon dioxide, butene, sec-butyl alcohol and acetone. The hydrolysis rate of sec-butyl acetate is small. When aminolysis occurs in a dilute alcohol solution at room temperature, 20% is converted into an amide in 120 hours. It reacts with benzene in the presence of boron trifluoride to form sec-butylbenzene. During the photochlorination reaction, chlorobutylacetate is produced. Among them, 1-methyl-2-chloropropyl acetate accounts for 66%, and other isomers account for 34%.
2. Stability[20] Stable
3. Incompatible substances[21] Strong oxidants, strong acids, strong bases
4. Polymerization hazards[22] No polymerization
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Gasoline antiknock agent is produced by the esterification of acetic acid and sec-butanol in the presence of sulfuric acid.
Refining method: often contains impurities such as water, acetic acid, sec-butanol and sec-butyl acetate isomers and homologues. During refining, first wash with sodium bicarbonate or saturated sodium carbonate solution, then wash with saturated aqueous sodium chloride solution, dry with anhydrous sodium sulfate or magnesium sulfate and then rectify.
Purpose
1. Mainly used for paint solvents, thinners, various vegetable oils and resin solvents. Also used in plastics and spice manufacturing. Gasoline antiknock agent.
2. Used as solvents, chemical reagents, and used to prepare spices. [24]

 微信扫一扫打赏
微信扫一扫打赏

