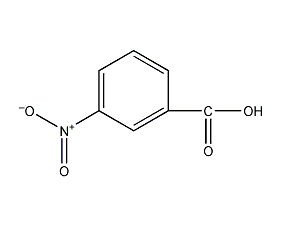
Structural formula
| Business number | 03EA |
|---|---|
| Molecular formula | C7H5NO4 |
| Molecular weight | 167 |
| label |
m-nitrobenzoic acid, 3-nitrobenzoic acid, m-nitrobenzoic acid, Rarechem Al Bo 0229, M-Nitrobenzoic Acid, Nitrobenzoic(3-) Acid |
Numbering system
CAS number:121-92-6
MDL number:MFCD00007251
EINECS number:204-508-5
RTECS number:DH5000000
BRN number:908644
PubChem ID:None
Physical property data
1. Properties: white or light yellow crystals, or crystalline powder with a bitter almond taste.
2. Density (g/mL, 25/4℃): 1.494
3. Melting point (℃): 142℃
4. Solubility: Insoluble in cold water, more soluble in hot water, soluble in ethanol, ether, chloroform, methanol and acetone, slightly soluble in benzene. Carbon disulfide and petroleum ether.
Toxicological data
1. Acute toxicity: rat peritoneal cavity LD50: 670mg/kg
rat intravenous injection LD50: 680mg/kg
rat gastrointestinal LD50: 1820mg/ kg
Mouse peritoneal cavity LD50: 610mg/kg
Mouse intravenous injection LD50: 640mg/kg
Mouse gastrointestinal LD50: 1290mg/ kg
2. Other multiple dose toxicity: Rat oral TDLO: 11375mg/kg/13W-C
Mouse oral TDLO: 54600mg/kg/13W-C
p>
3. Mutagenic toxicity: Salmonella mutation test system: 100ug/plate
Bacillus subtilis DNA patch test system: 500ug/disc
Ecological data
None
Molecular structure data
1. Molar refractive index: 39.72
2. Molar volume (cm3/mol): 113.8
3. Isotonic specific volume (90.2K): 324.8
4. Surface tension (dyne/cm): 66.4
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 15.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 83.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 198
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is acidic and irritating to the skin.
Storage method
Pack tightly and store in a cool and ventilated place. Store and transport according to the regulations of organic drugs.
Synthesis method
In industry, benzoic acid is used as raw material and is nitrated with sodium nitrate in the presence of sulfuric acid. The yield can reach 60%. If benzoyl is esterified to methyl benzoate with methanol in the presence of sulfuric acid, then nitrated with mixed acid, and then hydrolyzed, production by this method is a little troublesome, but the yield can be close to 70%. It can also be obtained by direct nitrification with mixed acid. The yield is around 60%.
Purpose
It is used in the pharmaceutical industry to produce cholecystic acid, acetoiodobenzoic acid, etc. It is also an intermediate for photosensitive materials, functional pigments and organic synthesis. It is also a reagent for measuring thorium and alkaloids.

 微信扫一扫打赏
微信扫一扫打赏

