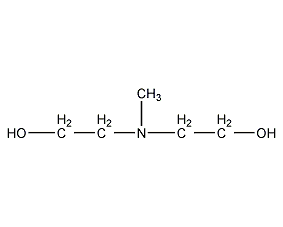
Structural formula
| Business number | 02S0 |
|---|---|
| Molecular formula | C5H13NO2 |
| Molecular weight | 119.16 |
| label |
2,2′-methyliminoethanolamine, N,N-bis(2-hydroxyethyl)methylamine, Methyldiethanolamine, 2,2′-Methyliminodiethanol, N,N-Bis(2-hydroxyethyl)methylamine, MDEA |
Numbering system
CAS number:105-59-9
MDL number:MFCD00002848
EINECS number:203-312-7
RTECS number:KL7525000
BRN number:1734441
PubChem ID:None
Physical property data
1. Properties: colorless or dark yellow oily liquid.
2. Density (g/mL, 25℃): 1.0377
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -21
5. Boiling point (ºC, normal pressure): 247.2
6. Boiling point (ºC, mmHg): Undetermined
7. Refractive index: 1.4678
8. Flash point (ºC): 127
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC):
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Not determined Determined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa ): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Miscible with water and alcohol. Slightly soluble in ether.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 502mgREACTION SEVERITY, slight reaction; 2. Acute toxicity: rat oral LD50: 4780mg/kg; mouse peritoneal cavity LD50: 500mg/kg; rabbit skin contact LD50: 5990μL/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 32.00
2. Molar volume (cm3/mol): 113.3
3. Isotonic specific volume (90.2K ): 290.2
4. Surface tension (dyne/cm): 43.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 12.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -1.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 43.7
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 43.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants.
2.The oral LD50 of this product in rats is 4.78mg/kg.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Keep away from light. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of formaldehyde and diethanolamine. Add formic acid to the reaction pot and heat to boiling. Add the mixture of formaldehyde and diethanolamine dropwise while stirring. The dripping is completed in about 1 hour. Maintain the temperature at 90-98°C and continue to reflux for 4 hours. Then perform vacuum distillation and collect the 120-130°C (0.53kPa) fraction to obtain N-methyldiethanolamine with a yield of 85%.
2. Obtained from the reaction of methylamine and ethylene oxide. Keep the temperature below 30°C, and pass ethylene oxide gas into the 20% methylamine solution to perform the addition reaction until the relative density of the reaction solution reaches 1.025. Stir for 15 minutes, and the end point is when the relative density remains unchanged. Recover methylamine under normal pressure to 103°C. After distilling water under reduced pressure, collect the 119-170°C (4.67kPa) fraction. That is the finished product. The yield is 72%. Raw material consumption quota: ethylene oxide 950kg/t, methylamine (40%) 870kg/t. In addition, N-methyldiethanolamine can also be produced by using formaldehyde and cyanoethanol as raw materials through catalytic hydrogenation.
![]()
Purpose
Mainly used as emulsifier and acid gas absorbent, and also used as an intermediate for anti-tumor drugs.

 微信扫一扫打赏
微信扫一扫打赏

