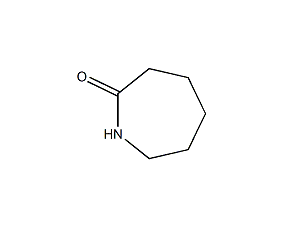
Structural formula
| Business number | 02S1 |
|---|---|
| Molecular formula | C6H11NO |
| Molecular weight | 113.16 |
| label |
2-Ketohexamethyleneimine, 2-Oxohexamethyleneimine, Aza-2-cycloheptanone, Nitrogen-containing compound solvents, Heterocyclic compounds |
Numbering system
CAS number:105-60-2
MDL number:MFCD00006936
EINECS number:203-313-2
RTECS number:CM3675000
BRN number:106934
PubChem number:24852860
Physical property data
1. Properties: White leaf-shaped crystals with a mint aroma.
2. Density (g/mL, 77/4℃): 1.023
3. Melting point (ºC): 69.2
4. Boiling point (ºC, Normal pressure): 268
5. Boiling point (ºC, 6.67KPa): 180
6. Refractive index (31ºC): 1.4965
7. Refractive index (40ºC): 1.4935
8. Viscosity (mPa·s, 70ºC): 19.7
9. Viscosity (mPa·s, 78ºC): 9
10. Flash point (ºC, open): 125
11. Autoignition point or ignition temperature (ºC): 375
12. Vapor pressure (kPa, 100ºC): 0.39
13. Vapor pressure (kPa, 180ºC): 6.67
14. Heat of evaporation (KJ/mol): 54.8
15. Heat of fusion (KJ/mol) ): 16.14
16. Heat of combustion (KJ/mol, standard conditions): 360.74
17. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.49
18. Solubility (%, 25ºC, water): 84
19. Heat of polymerization (KJ/mol): 83.7
20. Explosion limit (% , V/V): 8
21. Lower explosion limit (%, V/V): 1.4
22. Solubility: soluble in water, petroleum hydrocarbons, cyclohexene, Benzene, toluene, ethanol, ether, tetrahydrofurfural, dimethylformamide and chlorinated hydrocarbons.
Toxicological data
1. Acute toxicity: rat oral LD50: 1210mg/kg; mouse oral LD50: 930mg/kg; : 1410mg/kg
2. It is a low-toxic substance, but frequent exposure may cause symptoms such as headache, dizziness, fatigue, memory loss, sleep disorders and other neurasthenia symptoms. Symptoms such as epistaxis, dry nose, upper respiratory tract inflammation, and heartburn may occur at a concentration of 61 mg/m3. Caprolactam monomer is highly hygroscopic and easily soluble in sebum, so it can be absorbed by the skin. It is easy to cause skin damage when in contact, such as smooth and dry skin, thickened stratum corneum, chapped skin, desquamation, etc., and sometimes full-scale skin damage may occur.Sexual dermatitis. The olfactory threshold concentration is 0.3mg/m3, and the maximum allowable concentration in the workplace is 10mg/m3.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 31.14
2. Molar volume (cm3/mol): 116.7
3. Isotonic specific volume (90.2K ): 274.1
4. Surface tension (dyne/cm): 30.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 12.34
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 90.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants and alkali.
Chemical properties: It is easily hydrolyzed by heating in the presence of acid or alkali to generate εaminocaproic acid. When heated in the presence of water and amino acids, ring-opening polymerization occurs and a linear polymer compound (nylon-6) is generated. ε-Caprolactam turns yellow when in liquid state when in contact with air.
2.This product is toxic. Long-term inhalation can cause chronic poisoning, such as neurasthenia, dizziness, headache, nosebleeds and respiratory tract inflammation. It is corrosive to the skin, so direct contact should be avoided. Mice inhaled LC50450mg/m3. The maximum allowable concentration in the air is 10mg/m3. The production site must have forced ventilation. Operators must wear labor protection equipment.
Storage method
Packed in plastic bags, heat-sealed, and nylonwoven bags or kraft paper bags, 25kg per bag. Store in a dry and clean warehouse, protected from fire, moisture, heat and sun. Shelf life is three months.
Synthesis method
(1) Using phenol as raw material, it is obtained by Beckmann rearrangement of cyclohexanol, cyclohexanone and cyclohexanone oxime under acidic conditions;
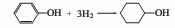
(2) Using cyclohexane as raw material, synthesized by photonitrosation method;
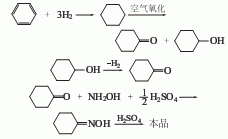
(3) Using toluene as raw material, it is synthesized by Snia method. In addition, furfural or acetylene can also be used as raw materials for synthesis.
Refining method: Take 250g of colored ε-caprolactam, add 750g of nitromethane and 6ml of 30% hydrogen peroxide, heat for 1 hour, and then distill under reduced pressure in the presence of 0.5% sodium hydroxide. The pure product obtained will not change color when exposed to light and air. Other refining methods include: vacuum distillation, and the distillate is recrystallized with petroleum ether or acetone and then distilled again.
Purpose
Widely used in gears, bearings, pipes, medical equipment and electrical insulation materials, etc. In the pharmaceutical industry, it is used as a raw material for the synthesis of drugs. The organic industry is used in the manufacture of lysine, etc. Used as solvent in chemical production. In analytical chemistry, it is used as gas chromatography stationary liquid, etc. Used to prepare caprolactam resin, polycaprolactam fiber and artificial leather, etc.

 微信扫一扫打赏
微信扫一扫打赏

