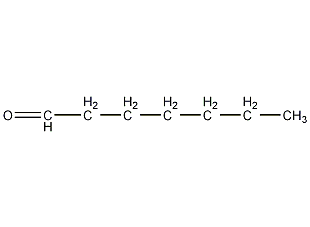
Structural formula
| Business number | 0345 |
|---|---|
| Molecular formula | C7H14O |
| Molecular weight | 114.19 |
| label |
Persaldehyde, n-Heptanal, seven-carbon aldehyde, Heptaldehyde, Enanthalelehyde, Oenanthaldehyde, n-Heptanal, food additives, Flavor enhancer |
Numbering system
CAS number:111-71-7
MDL number:MFCD00007028
EINECS number:203-898-4
RTECS number:MI690000
BRN number:1560236
PubChem number:24901128
Physical property data
1. Properties: colorless oily liquid with fruity aroma and hygroscopicity. [1]
2. Melting point (℃): -43[2]
3. Boiling point (℃): 153[3]
4. Relative density (water = 1): 0.82[4]
5. Relative vapor Density (air=1): 3.9[5]
6. Saturated vapor pressure (kPa): 0.4 (25℃)[6]
7. Critical pressure (MPa): 3.16[7]
8. Octanol/water partition coefficient: 1.99[8]
9. Flash point (℃): 35[9]
10. Ignition temperature (℃): 197[10 ]
11. Solubility: Slightly soluble in water, miscible in ethanol and ether, soluble in fixed oil, slightly soluble in carbon tetrachloride. [11]
12. Relative density (20℃, 4℃): 0.8174
13. Relative density (25℃, 4℃): 0.8132
14. Refractive index at room temperature (n25): 1.4094
15. Critical density (g·cm-3) : 0.263
16. Critical volume (cm3·mol-1): 434
17. Critical compression factor: 0.267
18. Eccentricity factor: 0.487
19. Solubility parameter (J·cm-3)0.5: 17.944
20.van der Waals area (cm2·mol-1): 1.124×1010
21. van der Waals volume (cm3·mol-1): 80.000
22. Gas phase standard heat of combustion (enthalpy) (kJ·mol-1): -4491.5
23. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -263.5
24. Gas phase standard entropy (J·mol-1·K-1): 461.66
25. Gas phase standard Free energy of formation (kJ·mol-1): -86.5
26. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 ): -4443.8
27. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -311.5
28. Liquid phase standard Entropy (J·mol-1·K-1): 348.5
29. Liquid phase standard formation free energy (kJ·mol -1): -100.4
30. Liquid phase standard hot melt (J·mol-1·K-1): 248.5
Toxicological data
1. Acute toxicity[12]
LD50: 3200mg/kg (rat oral); 25000mg/kg (mouse oral Oral); >5g/kg (rabbit transdermal)
LC5The primary or secondary alcohol can react completely within 5 to 15 minutes at 25°C, and at the same time, a brown-black chromium trioxide-pyridine reduction product is precipitated. The supernatant was decanted, and the precipitate was washed thoroughly with methylene chloride. Combine the dichloromethane solutions, wash with dilute hydrochloric acid, sodium bicarbonate, water, etc. in sequence to remove pyridine and chromium salts, or filter directly with filter aids, or separate with a chromatographic column, and evaporate the solvent to obtain the corresponding product. [21]
4. Preparation method:
![]()
In a reaction bottle equipped with a stirrer and thermometer, add 11g of silver p-toluenesulfonate ① dissolved in 100mL of acetonitrile. , cool to 0~5°C, add 7.0g (31mmol) of 1-iodoheptane (2), and stir and react at room temperature overnight in the dark. The reaction mixture was poured into ice water and extracted with diethyl ether. After drying the ether layer, the solvent was removed to obtain an oily substance. Add 20g of sodium bicarbonate and 150mL of DMSO to another reaction bottle, and add nitrogen gas to keep bubbling. Add the above oil and heat to 150℃ (sometimes it will smoke). After 3 minutes, cool quickly to room temperature. The generated aldehyde was reacted with 2,4-dinitrophenylhydrazine to generate the corresponding phenylhydrazone, and 6.9g of phenylhydrazone was obtained with a yield of 70% and mp of 106~107°C. When standard samples are mixed, the melting point of the mixture remains unchanged. Note: ① The preparation method is as follows: add equal moles of silver oxide and p-toluenesulfonic acid monohydrate into acetonitrile, stir and react for 30 minutes in the dark, and filter. The acetonitrile was evaporated, dried at 65°C, and set aside. [22]
Purpose
1. Because it comes from castor oil or one of the cracked products of ricinoleic acid methyl ester, it is mainly used as a raw material for the synthesis of spices-α-amylcinnamic aldehyde and corresponding homologous derivatives. The dosage for different types of edible flavors is 1.2~4.9mg/kg. Used as soap flavor, detergent flavor, perfume flavor, cream flavor, etc.
2. An important raw material for the synthesis of spices, and also a raw material for pharmaceuticals, organic synthesis and rubber products. [20]

 微信扫一扫打赏
微信扫一扫打赏

