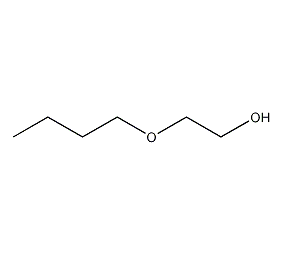
Structural formula
| Business number | 0348 |
|---|---|
| Molecular formula | C6H14O2 |
| Molecular weight | 118.17 |
| label |
Butyl glycol ether, Ethylene glycol butyl ether, Butoxyethanol, Butyl cellosolve, Hydroxyethyl Butyl Ether, Ethylene glycol butyl ether, Glycol ether, Butoxyethanol, Butyl cellosolve, 2-Hydroxyethyl butyl ether, Butyl cellosolve, paint stripper, D, metal detergent, delubricating agent, car engine detergent, dry cleaning solvent, drug extractants, alcohol solvent |
Numbering system
CAS number:111-76-2
MDL number:MFCD00002884
EINECS number:203-905-0
RTECS number:KJ8575000
BRN number:1732511
PubChem number:24855520
Physical property data
1. Appearance: colorless transparent liquid
2. Boiling point (ºC, 101.3kPa): 168.4
3. Melting point (ºC): -74.8
4. Relative density (g/mL, 20/4ºC): 0.9015
5. Relative density (g/mL, 27/4ºC): 0.89460
6. Relative steam Density (g/mL, air=1): 4.07
7. Refractive index (n20D): 1.4187
8. Viscosity (mPa·s, 25ºC): 3.15
9. Viscosity (mPa·s, 60ºC): 1.51
10. Flash point (ºC, closed): 60
11. Flash point (ºC, open): 74
12. Fire point (ºC): 244
13. Evaporation Heat (KJ/mol, average): 48.99
14. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.34
15. Critical temperature (ºC) :370
16. Critical pressure (MPa): 3.27
17. Conductivity (S/m, 20ºC): 4.32×10-7
18. Vapor pressure (kPa, 94ºC): 6.67
19. Vapor pressure (kPa, 61ºC): 1.33
20. Vapor pressure�� (kPa, 25ºC): 0.11
21. Lower explosion limit (%,V/V,170ºC): 1.1
22. Upper explosion limit (%,V/V,180ºC ): 10.6
23. Body expansion coefficient (K-1, 20ºC): 0.00092
24. Solubility: soluble in water, acetone, benzene , ether, methanol, carbon tetrachloride and other organic solvents and mineral oil. It is completely miscible with water at about 46°C. Can dissolve natural resin, ethyl cellulose, nitrocellulose, alkyd resin, polyethylene glycol, polyvinyl acetate, grease and paraffin, etc.
25. Relative density (25℃, 4℃): 0.845786.4
26. Critical density (g·cm-3
sup>): 0.279
27. Critical volume (cm3·mol-1): 424
28. Critical Compression factor: 0.263
29. Eccentricity factor: 0.817
30. Liquid phase standard hot melt (J·mol-1·K– 1): 285.3
Toxicological data
1. Acute toxicity: rat oral LD50: 2500 mg/kg; mouse oral LC50: 1200mg/kg; rabbit dermal LD50: 0.56mL/kg
2. Stimulation data: Skin – Rabbit 500 mg Mild; Eyes – Rabbit 100 mg Severe.
3. This product is easily absorbed by the skin, and the maximum allowable concentration in the workplace is 240 mg/m3. The anesthetic effect of poisons is the main cause of animal death. Autopsy revealed pulmonary congestion, severe renal congestion, and hemoglobinuria. Instillation into the eyes of animals can cause pain, conjunctival irritation, and minor temporary damage to the cornea.
Ecological data
Olfaction threshold concentration in the air: 0.35ppmBOD5(Five-day biological oxygen demand): 0.71g(Oxygen)/g(Sample)(Helan Standard)COD(Chemical Oxygen Demand): 2.2g(Oxygen )/g(Sample)
Molecular structure data
1. Molar refractive index: 33.12
2. Molar volume (cm3/mol): 131.4
3. Isotonic specific volume (90.2K ): 307.2
4. Surface tension (dyne/cm): 29.8
5. Polarizability (10-24cm3): 13.13
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 37.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with air. Prohibited contact withstrong oxidants, strong acids, acid chlorides, acid anhydrides, and halogens.
2.andThis product has low toxicity. Non-corrosive to metals. It has the general chemical properties of alcohol.
3. Exist in mainstream smoke.
4. The acute oral LD50 of mice is 1.48g/kg. Excessive inhalation can lead to hemolysis, Hematuria, headache, vomiting, etc.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air.
2. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. It should not be stored in large quantities or for long periods of time.
3. Equipped with corresponding varieties and quantities of fire-fighting equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the addition of ethylene oxide and n-butanol. Add n-butanol to the diethyl ether solution of boron trifluoride at 20°C, and add ethylene oxide while stirring. As the reaction proceeds, the temperature automatically rises. After the temperature drops, leave it for three days. Neutralize with potassium hydroxide methanol solution to pH=8 to obtain crude product. Add a little p-aminophenol to the crude product and carry out fractionation, and collect the 166-170°C fraction to obtain the finished product. Industrial production can adopt a non-catalytic reaction method under high temperature and high pressure (reaction temperature 180-250°C, pressure 2.1-4.6MPa), and the reaction lasts for 6 hours. Alkaline catalysts can also be used at near normal pressure and lower temperatures.
![]()
Refining method: easy to contain impurities Water, polymer of butyl glycol, ethylene glycol and trace amounts of acidic substances, etc. It can be refined by fractionation or by drying with anhydrous calcium chloride and anhydrous sodium sulfate and then fractionating.
Purpose
1. This product is mainly used as a high-boiling point solvent for paints, especially nitrocellulose spray paints, quick-drying paints, varnishes, enamels and paint strippers. It can prevent fogging, anti-wrinkling, and improve the gloss and fluidity of the paint film. It is also used as an inactive diluent for adhesives, metal detergent, paint remover, fiber wetting agent, pesticide dispersant, drug extractant, resin plasticizer, and organic synthesis intermediate. Reagents for the determination of iron and molybdenum. Auxiliary solvent to improve emulsification properties and dissolve mineral oil in soap.
2. Used as inactive diluent for adhesives, metal detergent, paint remover, fiber wetting agent, pesticide dispersant, Drug extractants, resin plasticizers and organic synthesis intermediates, etc. It is also used as a high boiling point solvent for paints, especially nitrocellulose spray paints. It can prevent fogging and wrinkles, and improve the gloss and fluidity of the paint film.
It is refined by drying calcium chloride and anhydrous sodium sulfate and then fractionating it.
Purpose
1. This product is mainly used as a high-boiling point solvent for paints, especially nitrocellulose spray paints, quick-drying paints, varnishes, enamels and paint strippers. It can prevent fogging, anti-wrinkling, and improve the gloss and fluidity of the paint film. It is also used as an inactive diluent for adhesives, metal detergent, paint remover, fiber wetting agent, pesticide dispersant, drug extractant, resin plasticizer, and organic synthesis intermediate. Reagents for the determination of iron and molybdenum. Auxiliary solvent to improve emulsification properties and dissolve mineral oil in soap.
2. Used as inactive diluent for adhesives, metal detergent, paint remover, fiber wetting agent, pesticide dispersant, Drug extractants, resin plasticizers and organic synthesis intermediates, etc. It is also used as a high boiling point solvent for paints, especially nitrocellulose spray paints. It can prevent fogging and wrinkles, and improve the gloss and fluidity of the paint film.

 微信扫一扫打赏
微信扫一扫打赏

