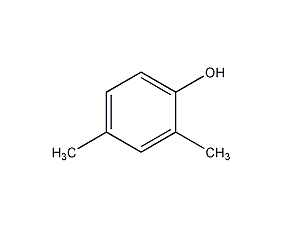
Structural formula
| Business number | 02S5 |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.16 |
| label |
4-Hydroxym-xylene, Meta-xylenol: 2,4-dimethylphenol, 4-Hydroxy-m-xylene, m-Xylenol, 2,4-Xylenol, preservative, Phenolic solvent |
Numbering system
CAS number:105-67-9
MDL number:MFCD00002233
EINECS number:203-321-6
RTECS number:ZE5600000
BRN number:636244
PubChem number:24862608
Physical property data
1. Properties: white crystal.
2. Boiling point (ºC, 101.3kPa): 211~212
3. Boiling point (ºC, 13.3kPa): 143
4. Boiling point (ºC , 2.67kPa): 105
5. Melting point (ºC): 22~23
6. Relative density (g/mL, 25/25ºC, liquid): 1.01601
7. Relative density (g/mL, 20/20ºC): 1.02017
8. Relative density (g/mL, 30/30ºC): 1.01186
9. Relative vapor density (g/mL, air=1): 4.23
10. Refractive index (14ºC): 1.5420
11. Flash point (ºC): 96
12. Heat of evaporation (KJ/mol): 47.18
13. Heat of generation (KJ/mol): 229.0
14. Heat of combustion (KJ/mol): 4351.38
15. Critical temperature (ºC): 434.4
16. Vapor pressure (kPa, 92ºC): 1.33
17. Solubility (%, 25ºC, water ): 0.5
18. Solubility: Hardly soluble in water, miscible with ethanol, chloroform, ether, benzene, etc. Soluble in sodium hydroxide aqueous solution.
19. Refractive index at room temperature (n25): 1.513474
20. Relative density (25℃, 4℃ ): 1.005940
21. Solubility parameter (J·cm-3)0.5: 21.153
22. van der Waals area (cm2·mol-1): 9.330×1010
23. van der Waals volume (cm3·mol-1): 73.780
24. Liquid phase standard heat of combustion (enthalpy) (kJ ·mol-1): -4348.5
25. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -228.7
26. Liquid phase standard hot melt (J·mol-1·K-1): 253.3
27. Gas phase Standard heat of combustion (enthalpy) (kJ·mol-1): -4414.4
28. Gas phase standard claims heat (enthalpy) (kJ·mol-1 sup>):-162.9
29. Gas phase standard entropy (J·mol-1·K-1): 397.83
30. Gas phase standard formation free energy (kJ· mol-1): -41.1
31. Gas phase standard hot melt (J·mol-1·K-1):156.10
Toxicological data
1. Acute toxicity: Rat oral LD50: 3200mg/kg; Rat inhalation LC: >30mg/m3; Rat skin contact LD50: 1040mg/kg; Mouse oral LD50: 809mg/kg; Mouse peritoneal cavity LD5 0 : 183mg/kg; Intravenous injection of mice LD50: 100mg/kg; 2. Other multiple dose toxicity: Rat oral TDLo: 6mg/kg/10D-I; Rat oral TDLo: 48600mg/kg/90D-I; 3. Chronic toxicity/carcinogenicity: Mouse skin contact TDLo: 16mg/kg/39W-I; 4. Steam is irritating to the eyes and respiratory mucosa, and 2,4-xylenol can be absorbed through the skin.
Ecological data
It is extremely harmful to water and toxic to fish. Do not let the product enter the water body.
Molecular structure data
1. Molar refractive index: 37.78
2. Molar volume (cm3/mol): 120.4
3. Isotonic specific volume (90.2K ): 297.5
4. Surface tension (dyne/cm): 37.2
5. Dielectric constant (F/m, 60ºC): 5.39
6 , Dipole moment (10-30C·m): 4.64
7, Polarizability: 14.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 90.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidizing agents. Corrosive and toxic. Can burn when exposed to open fire.
2. Exist in oriental tobacco leaves and mainstream smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Keep away from light. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from m-xylene through sulfonation, salting out, alkali fusion and acidification.
2. Tobacco: OR, 26.
Purpose
Used as a preservative and used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

